Probing the metabolic phenotype of breast cancer cells by multiple tracer stable isotope resolved metabolomics
- PMID: 28163219
- PMCID: PMC5540847
- DOI: 10.1016/j.ymben.2017.01.010
Probing the metabolic phenotype of breast cancer cells by multiple tracer stable isotope resolved metabolomics
Abstract
Breast cancers vary by their origin and specific set of genetic lesions, which gives rise to distinct phenotypes and differential response to targeted and untargeted chemotherapies. To explore the functional differences of different breast cell types, we performed Stable Isotope Resolved Metabolomics (SIRM) studies of one primary breast (HMEC) and three breast cancer cells (MCF-7, MDAMB-231, and ZR75-1) having distinct genotypes and growth characteristics, using 13C6-glucose, 13C-1+2-glucose, 13C5,15N2-Gln, 13C3-glycerol, and 13C8-octanoate as tracers. These tracers were designed to probe the central energy producing and anabolic pathways (glycolysis, pentose phosphate pathway, Krebs Cycle, glutaminolysis, nucleotide synthesis and lipid turnover). We found that glycolysis was not associated with the rate of breast cancer cell proliferation, glutaminolysis did not support lipid synthesis in primary breast or breast cancer cells, but was a major contributor to pyrimidine ring synthesis in all cell types; anaplerotic pyruvate carboxylation was activated in breast cancer versus primary cells. We also found that glucose metabolism in individual breast cancer cell lines differed between in vitro cultures and tumor xenografts, but not the metabolic distinctions between cell lines, which may reflect the influence of tumor architecture/microenvironment.
Keywords: (13)C(3)-glycerol; (13)C(3)-glycerol (PubChem CID:753); (13)C(5); (13)C(5),(15)N(2)-Glutamine (PubChem CID:5961); (13)C(6)-glucose; (13)C(6)-glucose (PubChem CID:5793); (13)C(8)-octanoate; (13)C(8)-octanoate (PubChem CID:11939); (15)N(2)-Gln; (PubChem CID:5927); 1D/2D NMR; ATP; Breast cancer metabolism; FT-ICR-MS; Mouse xenografts; Stable isotope-resolved metabolomics; UTP(PubChem CID:6133); alanine (PubChem CID:5950); aspartate (PubChem CID:5460541); glutamate (PubChem CID:5128032); lactate (PubChem CID:91435).
Copyright © 2017 International Metabolic Engineering Society. Published by Elsevier Inc. All rights reserved.
Figures
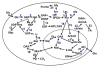

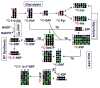
 ,
,
 represent 13C derived from OxPPP and NOxPPP, respectively. Ox PPP and NOx PPP: oxidative and non-oxidative branches of the pentose phosphate pathway, respectively; Glc: glucose; F6P: fructose-6-phosphate; GAP: glyceraldehyde-3-phosphate; Pyr: pyruvate; Lac: lactate; R5P: ribose-5-phosphate; X5P: xylulose-5-phosphate; Sed7P: sedoheptulose-7-phosphate; NXP: nucleotides; TK: transketolase; TA: transaldolase
represent 13C derived from OxPPP and NOxPPP, respectively. Ox PPP and NOx PPP: oxidative and non-oxidative branches of the pentose phosphate pathway, respectively; Glc: glucose; F6P: fructose-6-phosphate; GAP: glyceraldehyde-3-phosphate; Pyr: pyruvate; Lac: lactate; R5P: ribose-5-phosphate; X5P: xylulose-5-phosphate; Sed7P: sedoheptulose-7-phosphate; NXP: nucleotides; TK: transketolase; TA: transaldolase

 : 13C from pyruvate dehydrogenase-initiated Krebs cycle in A or from glutaminolysis+Krebs cycle in B;
: 13C from pyruvate dehydrogenase-initiated Krebs cycle in A or from glutaminolysis+Krebs cycle in B;
 : 13C from pyruvate carboxylase-initiated Krebs cycle ;
: 13C from pyruvate carboxylase-initiated Krebs cycle ;
 : 13C from glutaminolysis+Krebs cycle+malic enzyme (Le, Lane et al. 2012); DHO : dihydroorotate.
: 13C from glutaminolysis+Krebs cycle+malic enzyme (Le, Lane et al. 2012); DHO : dihydroorotate.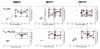


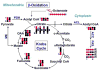

References
-
- ACS. Breast Cancer Facts & Figures 2015–2016. Atlanta: American Cancer Society; 2016. pp. 1–38.
-
- Bliss J, Kilburn L, Coleman R, Forbes J, Coates A, Jones S, Jassem J, Delozier T, Andersen J, Paridaens R, van de Velde C, Lønning P, Morden J, Reise J, Cisar L, Menschik T, Coombes R. Disease-related outcomes with long-term follow-up: an updated analysis of the intergroup exemestane study. J Clin Oncol. 2012;30:709–717. - PubMed
-
- Boren J, Cascante M, Marin S, Comin-Anduix B, Centelles JJ, Lim S, Bassilian S, Ahmed S, Lee WNP, Boros LG. Gleevec (ST1571) influences metabolic enzyme activities and glucose carbon flow toward nucleic acid and fatty acid synthesis in myeloid tumor cells. Journal of Biological Chemistry. 2001;276(41):37747–37753. - PubMed
-
- Boros LG, Puigjaner J, Cascante M, Lee WNP, Brandes JL, Bassilian S, Yusuf FI, Williams RD, Muscarella P, Melvin WS, Schirmer WJ. Oxythiamine and dehydroepiandrosterone inhibit the nonoxidative synthesis of ribose and tumor cell proliferation. Cancer Research. 1997;57(19):4242–4248. - PubMed
-
- Centelles JJ, Ramos-Montoya A, Lim S, Bassilian S, Boros LG, Marin S, Cascante M, Lee WNP. Metabolic profile and quantification of deoxyribose synthesis pathways in HepG2 cells. Metabolomics. 2007;3(2):105–111.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

