Destruction of biological particles using non-thermal plasma
- PMID: 28163377
- PMCID: PMC5281531
- DOI: 10.3164/jcbn.16-64
Destruction of biological particles using non-thermal plasma
Abstract
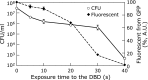
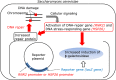
Mechanism of inactivation of bio-particles exposed to non-thermal plasma (NTP), namely, dielectric barrier discharge (DBD), and plasma jet (PJ), has been studied using E. coli, B. subtilis spore, S. cerevisiae and bacteriophages. States of different biological components were monitored during the course of inactivation. Analysis of green fluorescent protein, GFP, introduced into E. coli. or B. subtiles spore cells proved that radicals generated by NTP penetrate into microbes, destroying the cell membrane and finally damage the genes. We have evaluated the damage of the bacteriophages. Bacteriophage λ having double stranded DNA was exposed to DBD, then DNA was purified and subjected to in vitro DNA packaging reactions. The re-packaged phages consist of the DNA from discharged phages and brand-new coat proteins were proved to be active, indicating that the damage of coat proteins is responsible for inactivation. M13 phages having single stranded DNA were also examined with the same manner. In this case, damage to the DNA was as severe as that of the coat proteins. For practical applications, DBD showed very intense sterilization ability for B. Subtilis spore with the D-value of less than 10 s. This result indicates a possibility of application of NTP for quick sterilization.
Keywords: dielectric barrier discharge; non-thermal plasma; plasma jet; radical; sterilization.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
















References
-
- Mizuno A. Industrial applications of atmospheric non-thermal plasma in environmental remediation. Plasma Physics and Controlled Fusion. 2007;49:A1–A15.
-
- Mizuno A, Yamazaki Y, Ito H, Yoshida H. Ac energized ferroelectric pellet bed gas cleaner. IEEE Trans Ind Appl. 1992;28:535–540.
-
- Yasuda H, Hashimoto M, Rahman M, Takashima K, Mizuno A. States of biological components in bacteria and bacteriophages during inactivation by atmospheric dielectric barrier discharges. Plasma Process Polym. 2008;5:615–621.
-
- Fridman G, Brooks AD, Balasubramanian M, et al. Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria. Plasma Process Polym. 2007;4:370–375.
-
- Hashimoto M, Rahman MM, Tanino M, et al. Cell destruction by dielectric barrier discharge for real-time monitoring of bio-particles. Int J Plasma Environ Sci Technol. 2007;1:146–150.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

