Hydrazide-hydrazones as potential antimicrobial agents: overview of the literature since 2010
- PMID: 28163562
- PMCID: PMC5250660
- DOI: 10.1007/s00044-016-1756-y
Hydrazide-hydrazones as potential antimicrobial agents: overview of the literature since 2010
Abstract
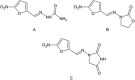
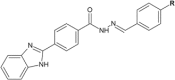
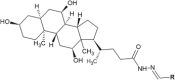
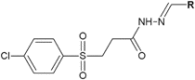
Hydrazide-hydrazone derivatives are present in many bioactive molecules and display a wide variety of biological activities, such as antibacterial, antitubercular, antifungal, anticancer, anti-inflammatory, anticonvulsant, antiviral, and antiprotozoal action. Therefore, many medicinal chemists synthesize various hydrazide-hydrazones and evaluate them for biological activities. Among biological properties of this class of compounds, antimicrobial activity is the most frequently encountered in scientific literature. This paper is focused on the overview of the literature findings of the last six years (2010-2016) covering the research on antimicrobial activity of hydrazide-hydrazone derivatives. This review may also serve as a useful guide for the development of new hydrazide-hydrazones as potential antimicrobial agents.
Keywords: Antibacterial activity; Antifungal activity; Antitubercular activity; Hydrazide–hydrazone; MIC.
Conflict of interest statement
The author declares that he has no competing interests.
Figures
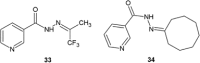
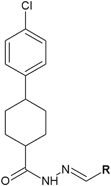
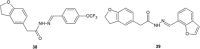
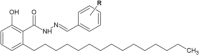
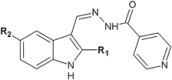
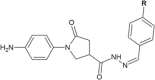
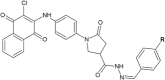
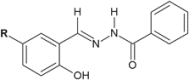
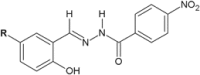
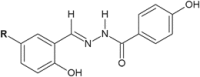
Similar articles
-
Updated Information on Antimicrobial Activity of Hydrazide-Hydrazones.Int J Mol Sci. 2021 Aug 30;22(17):9389. doi: 10.3390/ijms22179389. Int J Mol Sci. 2021. PMID: 34502297 Free PMC article. Review.
-
The application of hydrazones and hydrazide-hydrazones in the synthesis of bioactive azetidin-2-one derivatives: A mini review.Biomed Pharmacother. 2023 Jul;163:114853. doi: 10.1016/j.biopha.2023.114853. Epub 2023 May 11. Biomed Pharmacother. 2023. PMID: 37178574 Review.
-
Quinoline Hydrazide/Hydrazone Derivatives: Recent Insights on Antibacterial Activity and Mechanism of Action.ChemMedChem. 2023 Mar 1;18(5):e202200571. doi: 10.1002/cmdc.202200571. Epub 2023 Jan 20. ChemMedChem. 2023. PMID: 36617503 Review.
-
Hydrazides/hydrazones as antimicrobial and anticancer agents in the new millennium.Mini Rev Med Chem. 2013 Jun;13(7):971-87. Mini Rev Med Chem. 2013. PMID: 23621689 Review.
-
Searching for novel antimicrobial agents among hydrazide-hydrazones of 4-iodosalicylic acid.Biomed Pharmacother. 2022 Sep;153:113302. doi: 10.1016/j.biopha.2022.113302. Epub 2022 Jun 17. Biomed Pharmacother. 2022. PMID: 35724512
Cited by
-
Design, Synthesis, and In Vitro and In Vivo Bioactivity Studies of Hydrazide-Hydrazones of 2,4-Dihydroxybenzoic Acid.Int J Mol Sci. 2023 Dec 14;24(24):17481. doi: 10.3390/ijms242417481. Int J Mol Sci. 2023. PMID: 38139308 Free PMC article.
-
Targeting of the Mitochondrial TET1 Protein by Pyrrolo[3,2-b]pyrrole Chelators.Int J Mol Sci. 2022 Sep 16;23(18):10850. doi: 10.3390/ijms231810850. Int J Mol Sci. 2022. PMID: 36142763 Free PMC article.
-
Latent Tuberculosis: A Promising New Compound to Treat Non-Replicating and Intramacrophagic Mycobacteria.Biomedicines. 2022 Sep 26;10(10):2398. doi: 10.3390/biomedicines10102398. Biomedicines. 2022. PMID: 36289661 Free PMC article.
-
Constructing ROS-Responsive Supramolecular Gel with Innate Antibacterial Properties.Pharmaceutics. 2023 Aug 19;15(8):2161. doi: 10.3390/pharmaceutics15082161. Pharmaceutics. 2023. PMID: 37631375 Free PMC article.
-
Advancements in Hydrazide-Based HDAC Inhibitors: A Review of Recent Developments and Therapeutic Potential.J Med Chem. 2025 Jul 24;68(14):14171-14194. doi: 10.1021/acs.jmedchem.5c01677. Epub 2025 Jul 10. J Med Chem. 2025. PMID: 40637413 Free PMC article. Review.
References
-
- Al-Sharifi H, Patel HS. Synthesis, spectral investigation and biological evaluation of novel hydrazones derivative of substituted 1,2-dihydropyrimidine ring. Der Pharm Sin. 2012;3(3):305–311.
-
- Bala S, Uppal G, Kajal A, Kamboj S, Sharma V. Hydrazones as promising lead with diversity in bioactivity-therapeutic potential in present scenario. Int J Pharm Sci Rev Res. 2013;18(1):65–74.
-
- Chatterjee SN, Ghosh S. Mechanism of action of furazolidone: inter-strand cross-linking in DNA & liquid holding recovery of Vibrio cholerae cells. Indian J Biochem Biophys. 1979;16(3):125–130. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
