Dendritic Arborization Patterns of Small Juxtaglomerular Cell Subtypes within the Rodent Olfactory Bulb
- PMID: 28163674
- PMCID: PMC5247448
- DOI: 10.3389/fnana.2016.00127
Dendritic Arborization Patterns of Small Juxtaglomerular Cell Subtypes within the Rodent Olfactory Bulb
Abstract
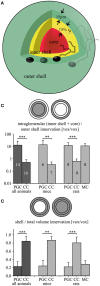
Within the glomerular layer of the rodent olfactory bulb, numerous subtypes of local interneurons contribute to early processing of incoming sensory information. Here we have investigated dopaminergic and other small local juxtaglomerular cells in rats and mice and characterized their dendritic arborization pattern with respect to individual glomeruli by fluorescent labeling via patching and reconstruction of dendrites and glomerular contours from two-photon imaging data. Dopaminergic neurons were identified in a transgenic mouse line where the expression of dopamine transporter (DAT) was labeled with GFP. Among the DAT+ cells we found a small short-axon cell (SAC) subtype featuring hitherto undescribed dendritic specializations. These densely ramifying structures clasped mostly around somata of other juxtaglomerular neurons, which were also small, non-dopaminergic and to a large extent non-GABAergic. Clasping SACs were observed also in wild-type mice and juvenile rats. In DAT+ SAC dendrites, single backpropagating action potentials evoked robust calcium entry throughout both clasping and non-clasping compartments. Besides clasping SACs, most other small neurons either corresponded to the classical periglomerular cell type (PGCs), which was never DAT+, or were undersized cells with a small dendritic tree and low excitability. Aside from the presence of clasps in SAC dendrites, many descriptors of dendritic morphology such as the number of dendrites and the extent of branching were not significantly different between clasping SACs and PGCs. However, a detailed morphometric analysis in relation to glomerular contours revealed that the dendrites of clasping SACs arborized mostly in the juxtaglomerular space and never entered more than one glomerulus (if at all), whereas most PGC dendrites were restricted to their parent glomerulus, similar to the apical tufts of mitral cells. These complementary arborization patterns might underlie a highly complementary functional connectivity. The morphometric approach may serve to differentiate also other subtypes of juxtaglomerular neurons, help to identify putative synaptic partners and thus to establish a more refined picture of glomerular network interactions during odor sensing.
Keywords: calcium imaging; dendrites; dendritic arborization analysis; dopaminergic neurons; juxtaglomerular cells; morphological reconstruction; subcellular compartments; two-photon imaging.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

