The New Synthetic H2S-Releasing SDSS Protects MC3T3-E1 Osteoblasts against H2O2-Induced Apoptosis by Suppressing Oxidative Stress, Inhibiting MAPKs, and Activating the PI3K/Akt Pathway
- PMID: 28163684
- PMCID: PMC5247634
- DOI: 10.3389/fphar.2017.00007
The New Synthetic H2S-Releasing SDSS Protects MC3T3-E1 Osteoblasts against H2O2-Induced Apoptosis by Suppressing Oxidative Stress, Inhibiting MAPKs, and Activating the PI3K/Akt Pathway
Abstract
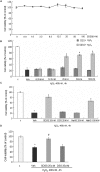
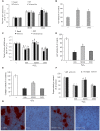
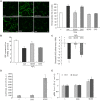
Reactive oxygen species (ROS) are important in osteoporosis development. Oxidative stress induces apoptosis of osteoblasts and arrest of their differentiation. Both Danshensu (DSS) and hydrogen sulfide (H2S) produce significant antioxidant effect in various systems. In this study, we synthesized SDSS, a novel H2S-releasing compound derived from DSS, and studied its antioxidant effect in an H2O2-induced MC3T3-E1 osteoblastic cell injury model. We first characterized the H2S releasing property of SDSS in both in vivo and in vitro models. HPLC chromatogram showed that intravenous injection of SDSS in adult rats released ADT-OH, a well proved H2S sustained-release moiety, within several minutes in the rat plasma. Using an H2S selective fluorescent probe, we further confirmed that SDSS released H2S in MC3T3-E1 osteoblastic cells. Biological studies revealed that SDSS had no significant toxic effect but produced protective effects against H2O2-induced MC3T3-E1 cell apoptosis. SDSS also reversed the arrest of cell differentiation caused by H2O2 treatment. This was caused by the stimulatory effect of SDSS on bone sialoprotein, runt-related transcription factor 2, collagen expression, alkaline phosphatase activity, and bone nodule formation. Further studies revealed that SDSS reversed the reduced superoxide dismutase activity and glutathione content, and the increased ROS production in H2O2 treated cells. In addition, SDSS significantly attenuated H2O2-induced activation of p38-, ERK1/2-, and JNK-MAPKs. SDSS also stimulated phosphatidylinositol 3-kinase/Akt signaling pathway. Blockade of this pathway attenuated the cytoprotective effect of SDSS. In conclusion, SDSS protects MC3T3-E1 cells against H2O2-induced apoptosis by suppressing oxidative stress, inhibiting MAPKs, and activating the phosphatidylinositol 3-kinase/Akt pathway.
Keywords: MAPK signaling; PI3K/AKT; hydrogen sulfide donating drugs; osteoblast; reactive oxygen species.
Figures



References
-
- Banerjee Mustafi S., Chakraborty P. K., Dey R. S., Raha S. (2009). Heat stress upregulates chaperone heat shock protein 70 and antioxidant manganese superoxide dismutase through reactive oxygen species (ROS), p38MAPK, and Akt. Cell Stress Chaperones 14 579–589. 10.1007/s12192-009-0109-x - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

