The Multifaceted Role of SNARE Proteins in Membrane Fusion
- PMID: 28163686
- PMCID: PMC5247469
- DOI: 10.3389/fphys.2017.00005
The Multifaceted Role of SNARE Proteins in Membrane Fusion
Abstract
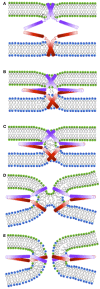
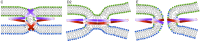
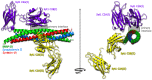
Membrane fusion is a key process in all living organisms that contributes to a variety of biological processes including viral infection, cell fertilization, as well as intracellular transport, and neurotransmitter release. In particular, the various membrane-enclosed compartments in eukaryotic cells need to exchange their contents and communicate across membranes. Efficient and controllable fusion of biological membranes is known to be driven by cooperative action of SNARE proteins, which constitute the central components of the eukaryotic fusion machinery responsible for fusion of synaptic vesicles with the plasma membrane. During exocytosis, vesicle-associated v-SNARE (synaptobrevin) and target cell-associated t-SNAREs (syntaxin and SNAP-25) assemble into a core trans-SNARE complex. This complex plays a versatile role at various stages of exocytosis ranging from the priming to fusion pore formation and expansion, finally resulting in the release or exchange of the vesicle content. This review summarizes current knowledge on the intricate molecular mechanisms underlying exocytosis triggered and catalyzed by SNARE proteins. Particular attention is given to the function of the peptidic SNARE membrane anchors and the role of SNARE-lipid interactions in fusion. Moreover, the regulatory mechanisms by synaptic auxiliary proteins in SNARE-driven membrane fusion are briefly outlined.
Keywords: SNAP-25; SNARE; fusion regulation; membrane fusion; protein-lipid interactions; synaptobrevin; syntaxin.
Figures






References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

