Vasoinhibin, an N-terminal Prolactin Fragment, Directly Inhibits Cardiac Angiogenesis in Three-dimensional Heart Culture
- PMID: 28163696
- PMCID: PMC5247450
- DOI: 10.3389/fendo.2017.00004
Vasoinhibin, an N-terminal Prolactin Fragment, Directly Inhibits Cardiac Angiogenesis in Three-dimensional Heart Culture
Abstract
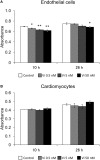
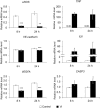
Vasoinhibins (Vi) are fragments of the growth hormone/prolactin (PRL) family and have antiangiogenic functions in many species. It is considered that Vi derived from PRL are involved in the pathogenesis of peripartum cardiomyopathy (PPCM). However, the pathogenic mechanism of PPCM, as well as heart angiogenesis, is not yet clear. Therefore, the aim of the present study is to clarify whether Vi act directly on angiogenesis inhibition in heart blood vessels. Endothelial cell viability was decreased by Vi treatment in a culture experiment. Furthermore, expression of proangiogenic genes, such as vascular endothelial growth factor, endothelial nitric oxide synthase, and VE-cadherin, were decreased. On the other hand, apoptotic factor gene, caspase 3, and inflammatory factor genes, tumor necrosis factor α and interleukin 6, were increased by Vi treatment. In three-dimensional left ventricular wall angiogenesis assay in mice, Vi treatment also inhibited cell migration, neovessel sprouting, and growth toward collagen gel. These data demonstrate that Vi treatment directly suppresses angiogenesis of the heart and support the hypothesis that Vi induce PPCM.
Keywords: angiogenesis; heart; peripartum cardiomyopathy; prolactin; vasoinhibin.
Figures


Similar articles
-
Vasoinhibin reduces joint inflammation, bone loss, and the angiogenesis and vasopermeability of the pannus in murine antigen-induced arthritis.Lab Invest. 2020 Aug;100(8):1068-1079. doi: 10.1038/s41374-020-0432-5. Epub 2020 Apr 27. Lab Invest. 2020. PMID: 32341517
-
From Bench to Bedside: Translating the Prolactin/Vasoinhibin Axis.Front Endocrinol (Lausanne). 2017 Dec 11;8:342. doi: 10.3389/fendo.2017.00342. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 29321761 Free PMC article. Review.
-
The HGR motif is the antiangiogenic determinant of vasoinhibin: implications for a therapeutic orally active oligopeptide.Angiogenesis. 2022 Feb;25(1):57-70. doi: 10.1007/s10456-021-09800-x. Epub 2021 Jun 7. Angiogenesis. 2022. PMID: 34097181 Free PMC article.
-
Regulation of blood vessels by prolactin and vasoinhibins.Adv Exp Med Biol. 2015;846:83-95. doi: 10.1007/978-3-319-12114-7_4. Adv Exp Med Biol. 2015. PMID: 25472535 Review.
-
Prolactin-derived vasoinhibins increase anxiety- and depression-related behaviors.Psychoneuroendocrinology. 2014 Jun;44:123-32. doi: 10.1016/j.psyneuen.2014.03.006. Epub 2014 Mar 21. Psychoneuroendocrinology. 2014. PMID: 24767626
Cited by
-
Prolactin levels and chronic kidney disease and the subsequent risk of cardiovascular events: A long term population based cohort study.Sci Rep. 2025 Feb 28;15(1):7198. doi: 10.1038/s41598-025-87783-1. Sci Rep. 2025. PMID: 40021736 Free PMC article.
-
Role of pregnancy hormones and hormonal interaction on the maternal cardiovascular system: a literature review.Clin Res Cardiol. 2019 Aug;108(8):831-846. doi: 10.1007/s00392-019-01441-x. Epub 2019 Feb 26. Clin Res Cardiol. 2019. PMID: 30806769 Review.
-
The 14-Kilodalton Human Growth Hormone Fragment a Potent Inhibitor of Angiogenesis and Tumor Metastasis.Int J Mol Sci. 2023 May 17;24(10):8877. doi: 10.3390/ijms24108877. Int J Mol Sci. 2023. PMID: 37240223 Free PMC article.
-
Modelling West Nile Virus and Usutu Virus Pathogenicity in Human Neural Stem Cells.Viruses. 2020 Aug 12;12(8):882. doi: 10.3390/v12080882. Viruses. 2020. PMID: 32806715 Free PMC article.
-
The role of prolactin/vasoinhibins in cardiovascular diseases.Animal Model Exp Med. 2023 Apr;6(2):81-91. doi: 10.1002/ame2.12264. Epub 2022 Aug 3. Animal Model Exp Med. 2023. PMID: 35923071 Free PMC article. Review.
References
-
- Struman I, Bentzien F, Lee HY, Mainfroid V, D’Angelo G, Goffin V, et al. Opposing actions of intact and N-terminal fragments of the human prolactin growth hormone family members on angiogenesis: an efficient mechanism for the regulation of angiogenesis. Proc Natl Acad Sci U S A (1999) 96:4.10.1073/pnas.96.4.1246 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

