Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis Form an "Operational Group B. amyloliquefaciens" within the B. subtilis Species Complex
- PMID: 28163698
- PMCID: PMC5247444
- DOI: 10.3389/fmicb.2017.00022
Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis Form an "Operational Group B. amyloliquefaciens" within the B. subtilis Species Complex
Abstract
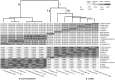
The plant growth promoting model bacterium FZB42T was proposed as the type strain of Bacillus amyloliquefaciens subsp. plantarum (Borriss et al., 2011), but has been recently recognized as being synonymous to Bacillus velezensis due to phylogenomic analysis (Dunlap C. et al., 2016). However, until now, majority of publications consider plant-associated close relatives of FZB42 still as "B. amyloliquefaciens." Here, we reinvestigated the taxonomic status of FZB42 and related strains in its context to the free-living soil bacterium DSM7T, the type strain of B. amyloliquefaciens. We identified 66 bacterial genomes from the NCBI data bank with high similarity to DSM7T. Dendrograms based on complete rpoB nucleotide sequences and on core genome sequences, respectively, clustered into a clade consisting of three tightly linked branches: (1) B. amyloliquefaciens, (2) Bacillus siamensis, and (3) a conspecific group containing the type strains of B. velezensis, Bacillus methylotrophicus, and B. amyloliquefaciens subsp. plantarum. The three monophyletic clades shared a common mutation rate of 0.01 substitutions per nucleotide position, but were distantly related to Bacillus subtilis (0.1 substitutions per nucleotide position). The tight relatedness of the three clusters was corroborated by TETRA, dDDH, ANI, and AAI analysis of the core genomes, but dDDH and ANI values were found slightly below species level thresholds when B. amyloliquefaciens DSM7T genome sequence was used as query sequence. Due to these results, we propose that the B. amyloliquefaciens clade should be considered as a taxonomic unit above of species level, designated here as "operational group B. amyloliquefaciens" consisting of the soil borne B. amyloliquefaciens, and plant associated B. siamensis and B. velezensis, whose members are closely related and allow identifying changes on the genomic level due to developing the plant-associated life-style.
Keywords: Bacillus amyloliquefaciens; Bacillus subtilis group; Bacillus taxonomy; average amino acid identity (AAI); average nucleotide identity (ANI); digital DNA–DNA hybridization; phylogenomics.
Figures



References
-
- Borriss R. (2011). Use of plant-associated Bacillus strains as biofertilizers and biocontrol agents, in Bacteria in Agrobiology: Plant Growth Response, ed Maheshwari D. K.(Heidelberg: Springer; ), 41–76.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

