The Social Life of Aeromonas through Biofilm and Quorum Sensing Systems
- PMID: 28163702
- PMCID: PMC5247445
- DOI: 10.3389/fmicb.2017.00037
The Social Life of Aeromonas through Biofilm and Quorum Sensing Systems
Abstract
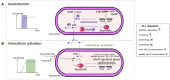
Bacteria of the genus Aeromonas display multicellular behaviors herein referred to as "social life". Since the 1990s, interest has grown in cell-to-cell communication through quorum sensing signals and biofilm formation. As they are interconnected, these two self-organizing systems deserve to be considered together for a fresh perspective on the natural history and lifestyles of aeromonads. In this review, we focus on the multicellular behaviors of Aeromonas, i.e., its social life. First, we review and discuss the available knowledge at the molecular and cellular levels for biofilm and quorum sensing. We then discuss the complex, subtle, and nested interconnections between the two systems. Finally, we focus on the aeromonad multicellular coordinated behaviors involved in heterotrophy and virulence that represent technological opportunities and applied research challenges.
Keywords: bacterial communities; biofilm; cooperation; coordination; multicellularity; quorum sensing; social life; virulence.
Figures



References
-
- Andreoli-Pinto T. J., Graziano K. U. (1999). Important aspects of the colonization of central venous catheter. Boll. Chim. Farm. 138, 19–23. - PubMed
-
- Balasubramanian V., Palanichamy S., Subramanian G., Rajaram R. (2012). Development of polyvinyl chloride biofilms for succession of selected marine bacterial populations. J. Environ. Biol. Acad. Environ. Biol. India 33, 57–60. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

