Activin A Modulates CRIPTO-1/HNF4 α+ Cells to Guide Cardiac Differentiation from Human Embryonic Stem Cells
- PMID: 28163723
- PMCID: PMC5253508
- DOI: 10.1155/2017/4651238
Activin A Modulates CRIPTO-1/HNF4 α+ Cells to Guide Cardiac Differentiation from Human Embryonic Stem Cells
Abstract
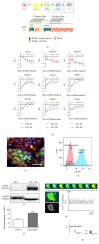
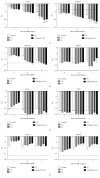
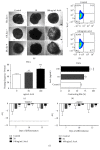
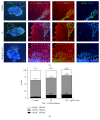
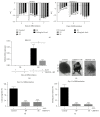
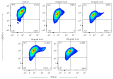
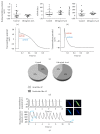
The use of human pluripotent stem cells in basic and translational cardiac research requires efficient differentiation protocols towards cardiomyocytes. In vitro differentiation yields heterogeneous populations of ventricular-, atrial-, and nodal-like cells hindering their potential applications in regenerative therapies. We described the effect of the growth factor Activin A during early human embryonic stem cell fate determination in cardiac differentiation. Addition of high levels of Activin A during embryoid body cardiac differentiation augmented the generation of endoderm derivatives, which in turn promoted cardiomyocyte differentiation. Moreover, a dose-dependent increase in the coreceptor expression of the TGF-β superfamily member CRIPTO-1 was observed in response to Activin A. We hypothesized that interactions between cells derived from meso- and endodermal lineages in embryoid bodies contributed to improved cell maturation in early stages of cardiac differentiation, improving the beating frequency and the percentage of contracting embryoid bodies. Activin A did not seem to affect the properties of cardiomyocytes at later stages of differentiation, measuring action potentials, and intracellular Ca2+ dynamics. These findings are relevant for improving our understanding on human heart development, and the proposed protocol could be further explored to obtain cardiomyocytes with functional phenotypes, similar to those observed in adult cardiac myocytes.
Conflict of interest statement
All the authors declare no potential conflict of interests.
Figures
Similar articles
-
Generation of functional murine cardiac myocytes from induced pluripotent stem cells.Circulation. 2008 Jul 29;118(5):507-17. doi: 10.1161/CIRCULATIONAHA.108.778795. Epub 2008 Jul 14. Circulation. 2008. PMID: 18625890
-
Embryonic stem cells differentiate in vitro into cardiomyocytes representing sinusnodal, atrial and ventricular cell types.Mech Dev. 1993 Nov;44(1):41-50. doi: 10.1016/0925-4773(93)90015-p. Mech Dev. 1993. PMID: 8155574
-
The acceleration of cardiomyogenesis in embryonic stem cells in vitro by serum depletion does not increase the number of developed cardiomyocytes.PLoS One. 2017 Mar 13;12(3):e0173140. doi: 10.1371/journal.pone.0173140. eCollection 2017. PLoS One. 2017. PMID: 28288171 Free PMC article.
-
Differential response of epiblast stem cells to Nodal and Activin signalling: a paradigm of early endoderm development in the embryo.Philos Trans R Soc Lond B Biol Sci. 2014 Dec 5;369(1657):20130550. doi: 10.1098/rstb.2013.0550. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25349457 Free PMC article. Review.
-
Human cardiomyocyte generation from pluripotent stem cells: A state-of-art.Life Sci. 2016 Jan 15;145:98-113. doi: 10.1016/j.lfs.2015.12.023. Epub 2015 Dec 10. Life Sci. 2016. PMID: 26682938 Review.
Cited by
-
Charting the Path: Navigating Embryonic Development to Potentially Safeguard against Congenital Heart Defects.J Pers Med. 2023 Aug 15;13(8):1263. doi: 10.3390/jpm13081263. J Pers Med. 2023. PMID: 37623513 Free PMC article. Review.
-
Stem Cell Technology in Cardiac Regeneration: A Pluripotent Stem Cell Promise.EBioMedicine. 2017 Feb;16:30-40. doi: 10.1016/j.ebiom.2017.01.029. Epub 2017 Jan 27. EBioMedicine. 2017. PMID: 28169191 Free PMC article. Review.
-
In the heart of the in vivo reprogramming.Stem Cell Investig. 2018 Oct 29;5:38. doi: 10.21037/sci.2018.10.03. eCollection 2018. Stem Cell Investig. 2018. PMID: 30498749 Free PMC article. No abstract available.
-
Long-term culture of patient-derived cardiac organoids recapitulated Duchenne muscular dystrophy cardiomyopathy and disease progression.Front Cell Dev Biol. 2022 Aug 11;10:878311. doi: 10.3389/fcell.2022.878311. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36035984 Free PMC article.
-
Stroma-derived IL-6, G-CSF and Activin-A mediated dedifferentiation of lung carcinoma cells into cancer stem cells.Sci Rep. 2018 Aug 1;8(1):11573. doi: 10.1038/s41598-018-29947-w. Sci Rep. 2018. PMID: 30069023 Free PMC article.
References
-
- Conlon F. L., Lyons K. M., Takaesu N., et al. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120(7):1919–1928. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

