Management of Infected Total Knee Arthroplasty by a New Innovative Customized Articulating Knee Spacer: An Early Experience
- PMID: 28164047
- PMCID: PMC5288616
- DOI: 10.13107/jocr.2250-0685.552
Management of Infected Total Knee Arthroplasty by a New Innovative Customized Articulating Knee Spacer: An Early Experience
Abstract
Introduction: Management of infected total knee arthroplasty (TKA) is a challenge to patient and surgeon alike. Two-stage exchange is the universally acclaimed method to tackle this problem. Various spacer devices are available for the first stage surgery for local delivery of antibiotics. Here, we report our experience with management of infected TKA patients with our indigenously designed and produced knee spacer.
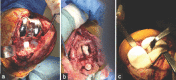
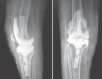
Case report: Between 2012 and 2013, 28 patients with infected total knee replacement (TKR) have been managed by our indigenous knee spacer. Minimal spacer-related complications and a stable knee joint with range of motion up to 100° were noted in these patients. After a mean period of 6-8 weeks, the spacer was removed and definitive TKR fixation done. At a mean follow-up of 4-months post second stage definitive surgery, patients were infection free with no evidence of recurrence of infection.
Conclusion: Our new innovative customized articulating knee spacer, which has intramedullary stem extension, has potential to significantly reduce spacer-related complications along with providing improved knee function.
Keywords: Infected total knee replacement; bone cement; knee spacer.
Conflict of interest statement
Conflict of Interest: Nil
Figures

Similar articles
-
Short-term Follow-up of Antibiotic-loaded Articulating Cement Spacers in Two-stage Revision of Infected Total Knee Arthroplasty: A Case Series.Orthop Surg. 2018 May;10(2):128-133. doi: 10.1111/os.12381. Epub 2018 May 17. Orthop Surg. 2018. PMID: 29770589 Free PMC article.
-
Two-stage approach to total knee arthroplasty using colistin-loaded articulating cement spacer for vancomycin-resistant Pseudomonas aeruginosa infection in an arthritic knee.Eur J Orthop Surg Traumatol. 2019 Jan;29(1):227-230. doi: 10.1007/s00590-018-2268-x. Epub 2018 Jun 18. Eur J Orthop Surg Traumatol. 2019. PMID: 29915953 Review.
-
Antibiotic-loaded articulating cement spacers in two-stage revision for infected total knee arthroplasty: individual antibiotic treatment and early results of 21 cases.Chin J Traumatol. 2012;15(4):212-21. Chin J Traumatol. 2012. PMID: 22863338
-
Functional interest of an articulating spacer in two-stage infected total knee arthroplasty revision.Orthop Traumatol Surg Res. 2014 Jun;100(4):409-12. doi: 10.1016/j.otsr.2014.01.010. Epub 2014 Apr 18. Orthop Traumatol Surg Res. 2014. PMID: 24746494
-
Comparison of infection eradication rate of using articulating spacers containing bio-inert materials versus all-cement articulating spacers in revision of infected TKA: a systematic review and meta-analysis.Arch Orthop Trauma Surg. 2019 May;139(5):695-707. doi: 10.1007/s00402-019-03121-x. Epub 2019 Mar 8. Arch Orthop Trauma Surg. 2019. PMID: 30850888
Cited by
-
Are Static Spacers Superior to Articulated Spacers in the Staged Treatment of Infected Primary Knee Arthroplasty? A Systematic Review and Meta-Analysis.J Clin Med. 2022 Aug 18;11(16):4854. doi: 10.3390/jcm11164854. J Clin Med. 2022. PMID: 36013091 Free PMC article. Review.
References
-
- Kuechle DK, Landon GC, Musher DM, Noble PC. Elution of vancomycin, daptomycin, and amikacin from acrylic bone cement. Clin Orthop Relat Res. 1991;264:302–308. - PubMed
-
- Goldman RT, Scuderi GR, Insall JN. 2-stage reimplantation for infected total knee replacement. Clin Orthop Relat Res. 1996;331:118–124. - PubMed
-
- Hebert CK, Williams RE, Levy RS, Barrack RL. Cost of treating an infected total knee replacement. Clin Orthop Relat Res. 1996;331:140–145. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
