FAK signalling controls insulin sensitivity through regulation of adipocyte survival
- PMID: 28165007
- PMCID: PMC5303880
- DOI: 10.1038/ncomms14360
FAK signalling controls insulin sensitivity through regulation of adipocyte survival
Abstract
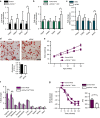
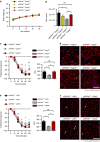
Focal adhesion kinase (FAK) plays a central role in integrin signalling, which regulates growth and survival of tumours. Here we show that FAK protein levels are increased in adipose tissue of insulin-resistant obese mice and humans. Disruption of adipocyte FAK in mice or in 3T3 L1 cells decreases adipocyte survival. Adipocyte-specific FAK knockout mice display impaired adipose tissue expansion and insulin resistance on prolonged metabolic stress from a high-fat diet or when crossed on an obese db/db or ob/ob genetic background. Treatment of these mice with a PPARγ agonist does not restore adiposity or improve insulin sensitivity. In contrast, inhibition of apoptosis, either genetically or pharmacologically, attenuates adipocyte death, restores normal adiposity and improves insulin sensitivity. Together, these results demonstrate that FAK is required for adipocyte survival and maintenance of insulin sensitivity, particularly in the context of adipose tissue expansion as a result of caloric excess.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
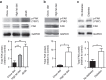
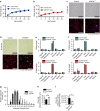

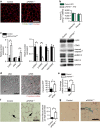
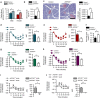
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

