New insights into the electrochemical behavior of acid orange 7: Convergent paired electrochemical synthesis of new aminonaphthol derivatives
- PMID: 28165049
- PMCID: PMC5292738
- DOI: 10.1038/srep41963
New insights into the electrochemical behavior of acid orange 7: Convergent paired electrochemical synthesis of new aminonaphthol derivatives
Abstract
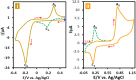
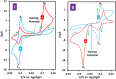
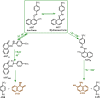
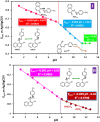
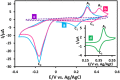
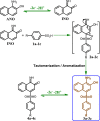
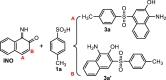
Electrochemical behavior of acid orange 7 has been exhaustively studied in aqueous solutions with different pH values, using cyclic voltammetry and constant current coulometry. This study has provided new insights into the mechanistic details, pH dependence and intermediate structure of both electrochemical oxidation and reduction of acid orange 7. Surprisingly, the results indicate that a same redox couple (1-iminonaphthalen-2(1H)-one/1-aminonaphthalen-2-ol) is formed from both oxidation and reduction of acid orange 7. Also, an additional purpose of this work is electrochemical synthesis of three new derivatives of 1-amino-4-(phenylsulfonyl)naphthalen-2-ol (3a-3c) under constant current electrolysis via electrochemical oxidation (and reduction) of acid orange 7 in the presence of arylsulfinic acids as nucleophiles. The results indicate that the electrogenerated 1-iminonaphthalen-2(1 H)-one participates in Michael addition reaction with arylsulfinic acids to form the 1-amino-3-(phenylsulfonyl)naphthalen-2-ol derivatives. The synthesis was carried out in an undivided cell equipped with carbon rods as an anode and cathode.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Electrochemical synthesis of sulfonamide derivatives based on the oxidation of 2,5-diethoxy-4-morpholinoaniline in the presence of arylsulfinic acids.J Org Chem. 2014 Jul 3;79(13):6326-9. doi: 10.1021/jo500812d. Epub 2014 Jun 19. J Org Chem. 2014. PMID: 24925683
-
Electroorganic synthesis of new benzofuro[2,3-d]pyrimidine derivatives.J Org Chem. 2002 Jul 12;67(14):5036-9. doi: 10.1021/jo0200623. J Org Chem. 2002. PMID: 12098334
-
Kinetic study of electrochemically induced michael reactions of o-quinones with Meldrum's acid derivatives. Synthesis of highly oxygenated catechols.J Org Chem. 2008 May 2;73(9):3428-34. doi: 10.1021/jo800115n. Epub 2008 Apr 9. J Org Chem. 2008. PMID: 18396907
-
Electrosynthesis of symmetric and highly conjugated benzofuran via a unique ECECCC electrochemical mechanism: evidence for predominance of electrochemical oxidation versus intramolecular cyclization.J Org Chem. 2007 May 11;72(10):3646-51. doi: 10.1021/jo062468b. Epub 2007 Apr 10. J Org Chem. 2007. PMID: 17419647
-
A new way for synthesis of phenoxazine and diphenoxazine derivatives via electrochemical method.Chem Pharm Bull (Tokyo). 2011;59(10):1209-13. doi: 10.1248/cpb.59.1209. Chem Pharm Bull (Tokyo). 2011. PMID: 21963628
Cited by
-
Efficient Photodegradation of Dyes from Single and Binary Aqueous Solutions Using Copper(II) Coordination Polymers.Molecules. 2025 Apr 8;30(8):1652. doi: 10.3390/molecules30081652. Molecules. 2025. PMID: 40333575 Free PMC article.
-
Electrochemical Sensing Strategies for Synthetic Orange Dyes.Molecules. 2024 Oct 24;29(21):5026. doi: 10.3390/molecules29215026. Molecules. 2024. PMID: 39519667 Free PMC article. Review.
-
An eco-friendly route for template-free synthesis of high specific surface area mesoporous CeO2 powders and their adsorption for acid orange 7.RSC Adv. 2019 Jul 18;9(39):22366-22375. doi: 10.1039/c9ra02294e. eCollection 2019 Jul 17. RSC Adv. 2019. PMID: 35519489 Free PMC article.
-
Convergent and Divergent Paired Electrodeposition of Metal-Organic Framework Thin Films.Sci Rep. 2019 Oct 4;9(1):14325. doi: 10.1038/s41598-019-50390-y. Sci Rep. 2019. PMID: 31586078 Free PMC article.
-
Activated Carbons Derived from Brewing Cereal Residues and Pineapple Peelings for Removal of Acid Orange 7 (AO7) Dye.Molecules. 2025 Feb 14;30(4):881. doi: 10.3390/molecules30040881. Molecules. 2025. PMID: 40005190 Free PMC article.
References
-
- Silva J. P. et al.. Adsorption of acid orange 7 dye in aqueous solutions by spent brewery grains. Sep. Purif. Technol. 40, 309–315 (2004).
-
- Zollinger H. Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments. (John Wiley & Sons, 2003).
-
- Hihara T., Okada Y. & Morita Z. Azo-hydrazone tautomerism of phenylazonaphthol sulfonates and their analysis using the semiempirical molecular orbital PM5 method. Dyes Pigm. 59, 25–41 (2003).
-
- Özen A. S., Doruker P. & Aviyente V. Effect of cooperative hydrogen bonding in azo-hydrazone tautomerism of azo dyes. J. Phys. Chem. A 111, 13506–13514 (2007). - PubMed
-
- Gordon P. F. & Gregory P. Organic Chemistry in Colour (Springer Science & Business Media, 2012).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

