Human T Cell Memory: A Dynamic View
- PMID: 28165397
- PMCID: PMC5371741
- DOI: 10.3390/vaccines5010005
Human T Cell Memory: A Dynamic View
Abstract
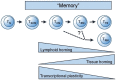
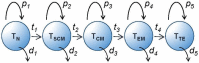
Long-term T cell-mediated protection depends upon the formation of a pool of memory cells to protect against future pathogen challenge. In this review we argue that looking at T cell memory from a dynamic viewpoint can help in understanding how memory populations are maintained following pathogen exposure or vaccination. For example, a dynamic view resolves the apparent paradox between the relatively short lifespans of individual memory cells and very long-lived immunological memory by focussing on the persistence of clonal populations, rather than individual cells. Clonal survival is achieved by balancing proliferation, death and differentiation rates within and between identifiable phenotypic pools; such pools correspond broadly to sequential stages in the linear differentiation pathway. Each pool has its own characteristic kinetics, but only when considered as a population; single cells exhibit considerable heterogeneity. In humans, we tend to concentrate on circulating cells, but memory T cells in non-lymphoid tissues and bone marrow are increasingly recognised as critical for immune defence; their kinetics, however, remain largely unexplored. Considering vaccination from this viewpoint shifts the focus from the size of the primary response to the survival of the clone and enables identification of critical system pinch-points and opportunities to improve vaccine efficacy.
Keywords: dynamics; immune memory; kinetics; proliferation; survival; turnover; vaccination; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

