DEK-targeting DNA aptamers as therapeutics for inflammatory arthritis
- PMID: 28165452
- PMCID: PMC5303823
- DOI: 10.1038/ncomms14252
DEK-targeting DNA aptamers as therapeutics for inflammatory arthritis
Abstract
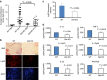
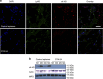
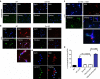
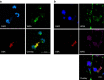
Novel therapeutics are required for improving the management of chronic inflammatory diseases. Aptamers are single-stranded RNA or DNA molecules that have recently shown utility in a clinical setting, as they can specifically neutralize biomedically relevant proteins, particularly cell surface and extracellular proteins. The nuclear chromatin protein DEK is a secreted chemoattractant that is abundant in the synovia of patients with juvenile idiopathic arthritis (JIA). Here, we show that DEK is crucial to the development of arthritis in mouse models, thus making it an appropriate target for aptamer-based therapy. Genetic depletion of DEK or treatment with DEK-targeted aptamers significantly reduces joint inflammation in vivo and greatly impairs the ability of neutrophils to form neutrophil extracellular traps (NETs). DEK is detected in spontaneously forming NETs from JIA patient synovial neutrophils, and DEK-targeted aptamers reduce NET formation. DEK is thus key to joint inflammation, and anti-DEK aptamers hold promise for the treatment of JIA and other types of arthritis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Comment in
-
Inflammation: Hit the DEK!Nat Rev Rheumatol. 2017 Apr;13(4):196-197. doi: 10.1038/nrrheum.2017.25. Epub 2017 Feb 23. Nat Rev Rheumatol. 2017. PMID: 28228649 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI062248/AI/NIAID NIH HHS/United States
- K01 AR055620/AR/NIAMS NIH HHS/United States
- R03 AR056748/AR/NIAMS NIH HHS/United States
- T32 AR007080/AR/NIAMS NIH HHS/United States
- F32 HL094017/HL/NHLBI NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- F31 CA150523/CA/NCI NIH HHS/United States
- T32 GM007863/GM/NIGMS NIH HHS/United States
- R01 AR038477/AR/NIAMS NIH HHS/United States
- K08 AR066569/AR/NIAMS NIH HHS/United States
- UM1 AI110557/AI/NIAID NIH HHS/United States
- R03 AR052482/AR/NIAMS NIH HHS/United States
- F30 CA210379/CA/NCI NIH HHS/United States
- T32 GM007544/GM/NIGMS NIH HHS/United States
- R01 DK109188/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

