Photo-driven redox-neutral decarboxylative carbon-hydrogen trifluoromethylation of (hetero)arenes with trifluoroacetic acid
- PMID: 28165474
- PMCID: PMC5303830
- DOI: 10.1038/ncomms14353
Photo-driven redox-neutral decarboxylative carbon-hydrogen trifluoromethylation of (hetero)arenes with trifluoroacetic acid
Abstract
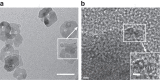
Catalytic oxidative C-H bond functionalization reactions that proceed without requiring stoichiometric amounts of external oxidants or pre-functionalized oxidizing reagents could maximize the atom- and step-economy in chemical syntheses. However, such a transformation remains elusive. Here, we report that a photo-driven catalytic process enables decarboxylative C-H trifluoromethylation of (hetero)arenes with trifluoroacetic acid as a trifluoromethyl source in good yields in the presence of an external oxidant in far lower than stoichiometric amounts (for example, 0.2 equivalents of Na2S2O8) using Rh-modified TiO2 nanoparticles as a photocatalyst, in which H2 release is an important driving force for the reaction. Our findings not only provide an approach to accessing valuable decarboxylative C-H trifluoromethylations via activation of abundant but inert trifluoroacetic acid towards oxidative decarboxylation and trifluoromethyl radical formation, but also demonstrate that a photo-driven catalytic process is a promising way to achieve external oxidant-free C-H functionalization reactions.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Dick A. R. & Sanford M. S. Transition metal catalyzed oxidative functionalization of carbon–hydrogen bonds. Tetrahedron 62, 2439–2463 (2006).
-
- Verma S., Baig R. B. N., Hanb C., Nadagouda M. N. & Varma R. S. Magnetic graphitic carbon nitride: application in C–H activation of amines. Chem. Commun. 51, 15554–15557 (2015). - PubMed
-
- Verma S., Baig R. B. N., Hanb C., Nadagouda M. N. & Varma R. S. Oxidative esterification via photocatalytic C–H activation. Green Chem. 18, 251–254 (2016).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

