A distinct innate lymphoid cell population regulates tumor-associated T cells
- PMID: 28165478
- PMCID: PMC5497996
- DOI: 10.1038/nm.4278
A distinct innate lymphoid cell population regulates tumor-associated T cells
Abstract
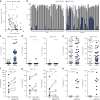
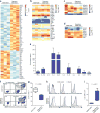
Antitumor T cells are subject to multiple mechanisms of negative regulation. Recent findings that innate lymphoid cells (ILCs) regulate adaptive T cell responses led us to examine the regulatory potential of ILCs in the context of cancer. We identified a unique ILC population that inhibits tumor-infiltrating lymphocytes (TILs) from high-grade serous tumors, defined their suppressive capacity in vitro, and performed a comprehensive analysis of their phenotype. Notably, the presence of this CD56+CD3- population in TIL cultures was associated with reduced T cell numbers, and further functional studies demonstrated that this population suppressed TIL expansion and altered TIL cytokine production. Transcriptome analysis and phenotypic characterization determined that regulatory CD56+CD3- cells exhibit low cytotoxic activity, produce IL-22, and have an expression profile that overlaps with those of natural killer (NK) cells and other ILCs. NKp46 was highly expressed by these cells, and addition of anti-NKp46 antibodies to TIL cultures abrogated the ability of these regulatory ILCs to suppress T cell expansion. Notably, the presence of these regulatory ILCs in TIL cultures corresponded with a striking reduction in the time to disease recurrence. These studies demonstrate that a previously uncharacterized ILC population regulates the activity and expansion of tumor-associated T cells.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
CD103+CD56+ ILCs Are Associated with an Altered CD8+ T-cell Profile within the Tumor Microenvironment.Cancer Immunol Res. 2025 Apr 2;13(4):527-546. doi: 10.1158/2326-6066.CIR-24-0151. Cancer Immunol Res. 2025. PMID: 40084939 Free PMC article.
-
Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: the peripheral blood immune cell profile.Cancer Immunol Immunother. 2019 Jun;68(6):1011-1024. doi: 10.1007/s00262-019-02343-7. Epub 2019 May 3. Cancer Immunol Immunother. 2019. PMID: 31053876 Free PMC article.
-
Innate lymphoid cells and cancer: Role in tumor progression and inhibition.Eur J Immunol. 2021 Sep;51(9):2188-2205. doi: 10.1002/eji.202049033. Epub 2021 Jul 11. Eur J Immunol. 2021. PMID: 34189723 Free PMC article. Review.
-
Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors.Cancer Res. 2013 Jun 15;73(12):3499-510. doi: 10.1158/0008-5472.CAN-13-0371. Epub 2013 Apr 16. Cancer Res. 2013. PMID: 23592754
-
The dual-functional capability of cytokine-induced killer cells and application in tumor immunology.Hum Immunol. 2015 May;76(5):385-91. doi: 10.1016/j.humimm.2014.09.021. Epub 2014 Oct 8. Hum Immunol. 2015. PMID: 25305457 Review.
Cited by
-
The Immune Landscape of Thyroid Cancer in the Context of Immune Checkpoint Inhibition.Int J Mol Sci. 2019 Aug 13;20(16):3934. doi: 10.3390/ijms20163934. Int J Mol Sci. 2019. PMID: 31412566 Free PMC article. Review.
-
Naturally Killing the Silent Killer: NK Cell-Based Immunotherapy for Ovarian Cancer.Front Immunol. 2019 Aug 9;10:1782. doi: 10.3389/fimmu.2019.01782. eCollection 2019. Front Immunol. 2019. PMID: 31456796 Free PMC article. Review.
-
Bone marrow infiltrated Lnc-INSR induced suppressive immune microenvironment in pediatric acute lymphoblastic leukemia.Cell Death Dis. 2018 Oct 11;9(10):1043. doi: 10.1038/s41419-018-1078-8. Cell Death Dis. 2018. PMID: 30310051 Free PMC article.
-
Harnessing Unconventional T Cells and Innate Lymphoid Cells to Prevent and Treat Hematological Malignancies: Prospects for New Immunotherapy.Biomolecules. 2022 May 27;12(6):754. doi: 10.3390/biom12060754. Biomolecules. 2022. PMID: 35740879 Free PMC article. Review.
-
ILC3 function as a double-edged sword in inflammatory bowel diseases.Cell Death Dis. 2019 Apr 8;10(4):315. doi: 10.1038/s41419-019-1540-2. Cell Death Dis. 2019. PMID: 30962426 Free PMC article. Review.
References
-
- Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 2016;13:143–158. - PubMed
-
- Callahan MK, Postow MA, Wolchok JD. Targeting T cell co-receptors for cancer therapy. Immunity. 2016;44:1069–1078. - PubMed
-
- Crome SQ, Lang PA, Lang KS, Ohashi PS. Natural killer cells regulate diverse T cell responses. Trends Immunol. 2013;34:342–349. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

