Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases
- PMID: 28165648
- PMCID: PMC5406532
- DOI: 10.1111/cas.13184
Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases
Abstract
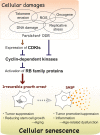
"Cellular senescence" is a state in which cells undergo irreversible cell cycle arrest in response to a variety of cellular stresses. Once cells senesce, they are strongly resistant to any mitogens, including oncogenic stimuli. Therefore, cellular senescence has been assumed to be a potent anticancer mechanism. Although irreversible cell-cycle arrest is traditionally considered the major characteristic of senescent cells, recent studies have revealed some additional functions. Most noteworthy is the increased secretion of various secretory proteins, such as inflammatory cytokines, chemokines, growth factors, and MMPs, into the surrounding extracellular fluid. These newly recognized senescent phenotypes, termed senescence-associated secretory phenotypes (SASPs), reportedly contribute to tumor suppression, wound healing, embryonic development, and even tumorigenesis promotion. Thus, SASPs appear to be beneficial or deleterious, depending on the biological context. As senescent cells are known to accumulate during the aging process in vivo, it is quite possible that their accumulation in aged tissues promotes age-associated functional decline and various diseases, including cancers, at least to some extent. Here, we focus on and discuss the functional and regulatory network of SASPs toward opening up new possibilities for controlling aging and aging-associated diseases.
Keywords: Aging; DNA damage response; immune response; senescence; tumorigenesis.
© 2017 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures




References
-
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res 1961; 25: 585–621. - PubMed
-
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 1965; 37: 614–36. - PubMed
-
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 2005; 120: 513–22. - PubMed
-
- Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science 2006; 311: 1257. - PubMed
-
- Takahashi A, Ohtani N, Yamakoshi K et al Mitogenic signalling and the p16INK4a‐Rb pathway cooperate to enforce irreversible cellular senescence. Nat Cell Biol 2006; 8: 1291–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

