Genome-wide DNA methylation associations with spontaneous preterm birth in US blacks: findings in maternal and cord blood samples
- PMID: 28165855
- PMCID: PMC5873359
- DOI: 10.1080/15592294.2017.1287654
Genome-wide DNA methylation associations with spontaneous preterm birth in US blacks: findings in maternal and cord blood samples
Abstract
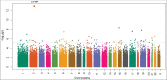
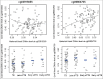
Preterm birth (PTB) affects one in six Black babies in the United States. Epigenetics is believed to play a role in PTB; however, only a limited number of epigenetic studies of PTB have been reported, most of which have focused on cord blood DNA methylation (DNAm) and/or were conducted in white populations. Here we conducted, by far, the largest epigenome-wide DNAm analysis in 300 Black women who delivered early spontaneous preterm (sPTB, n = 150) or full-term babies (n = 150) and replicated the findings in an independent set of Black mother-newborn pairs from the Boston Birth Cohort. DNAm in maternal blood and/or cord blood was measured using the Illumina HumanMethylation450 BeadChip. We identified 45 DNAm loci in maternal blood associated with early sPTB, with a false discovery rate (FDR) <5%. Replication analyses confirmed sPTB associations for cg03915055 and cg06804705, located in the promoter regions of the CYTIP and LINC00114 genes, respectively. Both loci had comparable associations with early sPTB and early medically-indicated PTB, but attenuated associations with late sPTB. These associations could not be explained by cell composition, gestational complications, and/or nearby maternal genetic variants. Analyses in the newborns of the 110 Black women showed that cord blood methylation levels at both loci had no associations with PTB. The findings from this study underscore the role of maternal DNAm in PTB risk, and provide a set of maternal loci that may serve as biomarkers for PTB. Longitudinal studies are needed to clarify temporal relationships between maternal DNAm and PTB risk.
Keywords: DNA methylation; epigenome-wide associations; maternal blood; spontaneous preterm birth.
Figures


Similar articles
-
Analysis of two birth tissues provides new insights into the epigenetic landscape of neonates born preterm.Clin Epigenetics. 2019 Feb 11;11(1):26. doi: 10.1186/s13148-018-0599-4. Clin Epigenetics. 2019. PMID: 30744680 Free PMC article.
-
DNA methylation mediates the effect of maternal smoking on offspring birthweight: a birth cohort study of multi-ethnic US mother-newborn pairs.Clin Epigenetics. 2021 Mar 4;13(1):47. doi: 10.1186/s13148-021-01032-6. Clin Epigenetics. 2021. PMID: 33663600 Free PMC article.
-
Comparison of DNA methylation profiles associated with spontaneous preterm birth in placenta and cord blood.BMC Med Genomics. 2019 Jan 3;12(1):1. doi: 10.1186/s12920-018-0466-3. BMC Med Genomics. 2019. PMID: 30606219 Free PMC article.
-
Gaining a deeper understanding of social determinants of preterm birth by integrating multi-omics data.Pediatr Res. 2021 Jan;89(2):336-343. doi: 10.1038/s41390-020-01266-9. Epub 2020 Nov 13. Pediatr Res. 2021. PMID: 33188285 Free PMC article. Review.
-
Meta-Analysis of Maternal and Fetal Transcriptomic Data Elucidates the Role of Adaptive and Innate Immunity in Preterm Birth.Front Immunol. 2018 May 9;9:993. doi: 10.3389/fimmu.2018.00993. eCollection 2018. Front Immunol. 2018. PMID: 29867970 Free PMC article.
Cited by
-
Association between one-carbon metabolism indices and DNA methylation status in maternal and cord blood.Sci Rep. 2018 Nov 15;8(1):16873. doi: 10.1038/s41598-018-35111-1. Sci Rep. 2018. PMID: 30442960 Free PMC article.
-
integRATE: a desirability-based data integration framework for the prioritization of candidate genes across heterogeneous omics and its application to preterm birth.BMC Med Genomics. 2018 Nov 19;11(1):107. doi: 10.1186/s12920-018-0426-y. BMC Med Genomics. 2018. PMID: 30453955 Free PMC article.
-
Preterm birth buccal cell epigenetic biomarkers to facilitate preventative medicine.Sci Rep. 2022 Mar 1;12(1):3361. doi: 10.1038/s41598-022-07262-9. Sci Rep. 2022. PMID: 35232984 Free PMC article.
-
Screening for spontaneous preterm birth and resultant therapies to reduce neonatal morbidity and mortality: A review.Semin Fetal Neonatal Med. 2018 Apr;23(2):126-132. doi: 10.1016/j.siny.2017.11.007. Epub 2017 Dec 9. Semin Fetal Neonatal Med. 2018. PMID: 29229486 Free PMC article. Review.
-
DNA Methylation Patterns of Glucocorticoid Pathway Genes in Preterm Birth Among Black Women.Biol Res Nurs. 2022 Oct;24(4):493-502. doi: 10.1177/10998004221099253. Epub 2022 May 5. Biol Res Nurs. 2022. PMID: 35512640 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
