Repositioning approved drugs for the treatment of problematic cancers using a screening approach
- PMID: 28166232
- PMCID: PMC5293254
- DOI: 10.1371/journal.pone.0171052
Repositioning approved drugs for the treatment of problematic cancers using a screening approach
Abstract
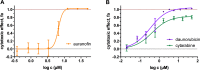
Advances in treatment strategies together with an earlier diagnosis have considerably increased the average survival of cancer patients over the last four decades. Nevertheless, despite the growing number of new antineoplastic agents introduced each year, there is still no adequate therapy for problematic malignancies such as pancreatic, lung and stomach cancers. Consequently, it is important to ensure that existing drugs used to treat other types of cancers, and potentially other diseases, are not overlooked when searching for new chemotherapy regimens for these problematic cancer types. We describe a screening approach that identifies chemotherapeutics for the treatment of lung and pancreatic cancers, based on drugs already approved for other applications. Initially, the 1280 chemically and pharmacologically diverse compounds from the Prestwick Chemical Library® (PCL) were screened against A549 (lung cancer) and PANC-1 (pancreatic carcinoma) cells using the PrestoBlue fluorescent-based cell viability assay. More than 100 compounds from the PCL were identified as hits in one or both cell lines (80 of them, being drugs used to treat diseases other than cancer). Selected PCL hits were further evaluated in a dose-response manner. Promising candidates for repositioning emanating from this study include antiparasitics, cardiac glycosides, as well as the anticancer drugs vorinostat and topotecan.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- WHO/Europe | Cancer. Available from: http://www.euro.who.int/en/health-topics/noncommunicable-diseases/cancer, accessed on 27.04.2016.
-
- John R, Ross H. The Global economic cost of cancer. Available from: http://www.cancer.org/acs/groups/content/@internationalaffairs/documents..., accessed on 27.04.2016.
-
- National Cancer Institute: What Is Cancer? Available from: http://www.cancer.gov/about-cancer/what-is-cancer, accessed on 27.04.2016.
-
- Bray F with Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Available from: http://globocan.iarc.fr, accessed on 28.04.2016.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

