Characterization of three-dimensional cancer cell migration in mixed collagen-Matrigel scaffolds using microfluidics and image analysis
- PMID: 28166248
- PMCID: PMC5293277
- DOI: 10.1371/journal.pone.0171417
Characterization of three-dimensional cancer cell migration in mixed collagen-Matrigel scaffolds using microfluidics and image analysis
Abstract
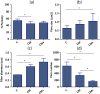
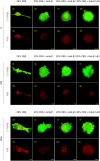
Microfluidic devices are becoming mainstream tools to recapitulate in vitro the behavior of cells and tissues. In this study, we use microfluidic devices filled with hydrogels of mixed collagen-Matrigel composition to study the migration of lung cancer cells under different cancer invasion microenvironments. We present the design of the microfluidic device, characterize the hydrogels morphologically and mechanically and use quantitative image analysis to measure the migration of H1299 lung adenocarcinoma cancer cells in different experimental conditions. Our results show the plasticity of lung cancer cell migration, which turns from mesenchymal in collagen only matrices, to lobopodial in collagen-Matrigel matrices that approximate the interface between a disrupted basement membrane and the underlying connective tissue. Our quantification of migration speed confirms a biphasic role of Matrigel. At low concentration, Matrigel facilitates migration, most probably by providing a supportive and growth factor retaining environment. At high concentration, Matrigel slows down migration, possibly due excessive attachment. Finally, we show that antibody-based integrin blockade promotes a change in migration phenotype from mesenchymal or lobopodial to amoeboid and analyze the effect of this change in migration dynamics, in regards to the structure of the matrix. In summary, we describe and characterize a robust microfluidic platform and a set of software tools that can be used to study lung cancer cell migration under different microenvironments and experimental conditions. This platform could be used in future studies, thus benefitting from the advantages introduced by microfluidic devices: precise control of the environment, excellent optical properties, parallelization for high throughput studies and efficient use of therapeutic drugs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Shaw LM, Tumor cell invasion assays. Methods Mol Biol. 2005; 294:97–105 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

