p38α regulates actin cytoskeleton and cytokinesis in hepatocytes during development and aging
- PMID: 28166285
- PMCID: PMC5293263
- DOI: 10.1371/journal.pone.0171738
p38α regulates actin cytoskeleton and cytokinesis in hepatocytes during development and aging
Abstract
Background: Hepatocyte poliploidization is an age-dependent process, being cytokinesis failure the main mechanism of polyploid hepatocyte formation. Our aim was to study the role of p38α MAPK in the regulation of actin cytoskeleton and cytokinesis in hepatocytes during development and aging.
Methods: Wild type and p38α liver-specific knock out mice at different ages (after weaning, adults and old) were used.
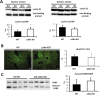
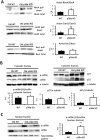
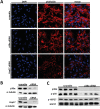
Results: We show that p38α MAPK deficiency induces actin disassembly upon aging and also cytokinesis failure leading to enhanced binucleation. Although the steady state levels of cyclin D1 in wild type and p38α knock out old livers remained unaffected, cyclin B1- a marker for G2/M transition- was significantly overexpressed in p38α knock out mice. Our findings suggest that hepatocytes do enter into S phase but they do not complete cell division upon p38α deficiency leading to cytokinesis failure and binucleation. Moreover, old liver-specific p38α MAPK knock out mice exhibited reduced F-actin polymerization and a dramatic loss of actin cytoskeleton. This was associated with abnormal hyperactivation of RhoA and Cdc42 GTPases. Long-term p38α deficiency drives to inactivation of HSP27, which seems to account for the impairment in actin cytoskeleton as Hsp27-silencing decreased the number and length of actin filaments in isolated hepatocytes.
Conclusions: p38α MAPK is essential for actin dynamics with age in hepatocytes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

