Spontaneous germinal centers and autoimmunity
- PMID: 28166685
- PMCID: PMC5669068
- DOI: 10.1080/08916934.2017.1280671
Spontaneous germinal centers and autoimmunity
Abstract
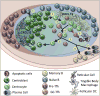
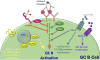
Germinal centers (GCs) are dynamic microenvironments that form in the secondary lymphoid organs and generate somatically mutated high-affinity antibodies necessary to establish an effective humoral immune response. Tight regulation of GC responses is critical for maintaining self-tolerance. GCs can arise in the absence of purposeful immunization or overt infection (called spontaneous GCs, Spt-GCs). In autoimmune-prone mice and patients with autoimmune disease, aberrant regulation of Spt-GCs is thought to promote the development of somatically mutated pathogenic autoantibodies and the subsequent development of autoimmunity. The mechanisms that control the formation of Spt-GCs and promote systemic autoimmune diseases remain an open question and the focus of ongoing studies. Here, we discuss the most current studies on the role of Spt-GCs in autoimmunity.
Keywords: Autoimmunity; ectopic germinal centers; self-tolerance; spontaneous germinal centers.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
