DEB TACE for Intermediate and advanced HCC - Initial Experience in a Brazilian Cancer Center
- PMID: 28166821
- PMCID: PMC5295188
- DOI: 10.1186/s40644-017-0108-6
DEB TACE for Intermediate and advanced HCC - Initial Experience in a Brazilian Cancer Center
Abstract
Background: According to Barcelona Clinic Liver Cancer classification transarterial chemoembolization is indicated in patients with Hepatocellular Carcinoma in the intermediate stage. Drug-eluting microspheres can absorb and release the chemotherapeutic agent slowly for 14 days after its intra-arterial administration. This type of transarterial chemoembolization approach appears to provide at least equivalent effectiveness with less toxicity.
Methods: This is a prospective, single-center study, which evaluated 21 patients with intermediate and advanced hepatocellular carcinoma who underwent transarterial chemoembolization with drug-eluting microspheres. The follow up period was 2 years. Inclusion criteria was Child-Pugh A or B liver disease patients, intermediate or advanced hepatocellular carcinoma and performance status equal or below 2. Transarterial chemoembolization with drug-eluting microspheres was performed at 2-month intervals during the first two sessions. The third and subsequent sessions were performed according to the image findings on follow-up, on a "demand schedule". Tumor response and time to progression were evaluated along the two-year follow up period.
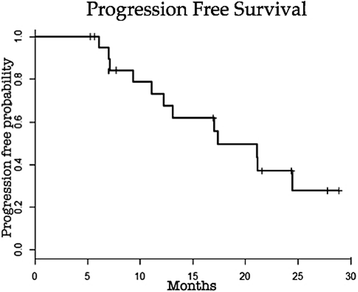
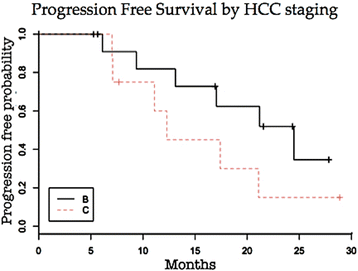
Results: Of the 21 patients 90% presented with liver cirrhosis, 62% had Barcelona Clinic Liver Cancer stage B and 38% had Barcelona Clinic Liver Cancer stage C hepatocellular carcinoma. Average tumor size was 6.9 cm. The average number of Transarterial chemoembolization with drug-eluting microspheres procedures was 3 with a total of 64 sessions. The predominant toxicity was mild. Liver function was not significantly affected in most patients. Two deaths occurred within 90 days after Transarterial chemoembolization with drug-eluting microspheres (ischemic hepatitis and hydropic decompensation). Technical success was achieved in 63 of 64 procedures. The mean hospital stay was 1.5 days. The progression free and overall survival at 1 and 2 years were 73.0% and 37.1%, 73.7% and 41.6%, respectively.
Conclusion: Transarterial chemoembolization with drug-eluting microspheres is able to deliver significant tumor response and progression free survival rate with acceptable toxicity. Larger studies are needed to identify exactly which subset of advanced hepatocellular patients may benefit from this treatment.
Trial registration: study ID ISRCTN16295622. Registered October 14th 2016. Retrospectively registered. Website registration: http://www.isrctn.com/ISRCTN16295622.
Figures
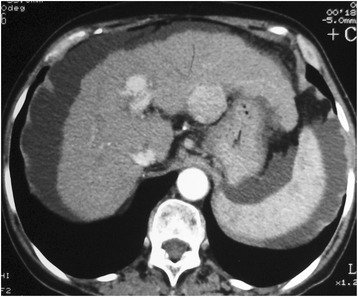
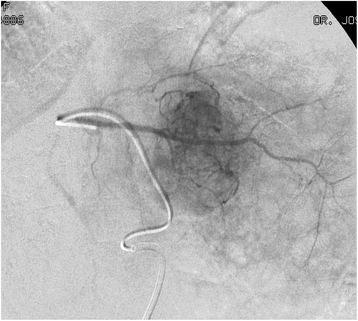
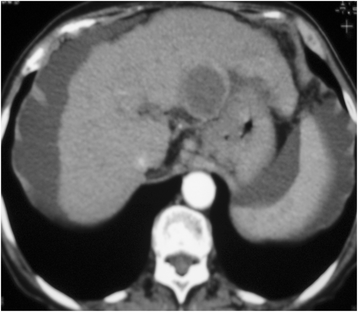
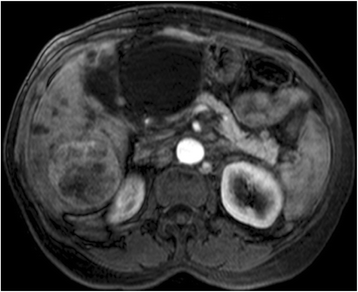
Similar articles
-
Transcatheter arterial chemoembolization with doxorubicin-eluting superabsorbent polymer microspheres in the treatment of hepatocellular carcinoma: midterm follow-up.J Vasc Interv Radiol. 2014 Feb;25(2):248-55.e1. doi: 10.1016/j.jvir.2013.10.017. Epub 2013 Dec 2. J Vasc Interv Radiol. 2014. PMID: 24295569
-
[Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEB-TACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma].Radiologia. 2011 May-Jun;53(3):246-53. doi: 10.1016/j.rx.2010.07.010. Epub 2011 Feb 4. Radiologia. 2011. PMID: 21295802 Spanish.
-
Efficacy of transarterial chemoembolization treatment with 30-60-μm microspheres in patients with hepatocellular carcinoma.Radiologie (Heidelb). 2024 Nov;64(Suppl 1):131-138. doi: 10.1007/s00117-024-01351-8. Epub 2024 Aug 7. Radiologie (Heidelb). 2024. PMID: 39112640 English.
-
Liver chemoembolization of hepatocellular carcinoma using TANDEM® microspheres.Future Oncol. 2018 Nov;14(26):2761-2772. doi: 10.2217/fon-2018-0237. Epub 2018 Jun 28. Future Oncol. 2018. PMID: 29953255 Review.
-
Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: Current state of the art.World J Gastroenterol. 2018 Jan 14;24(2):161-169. doi: 10.3748/wjg.v24.i2.161. World J Gastroenterol. 2018. PMID: 29375202 Free PMC article. Review.
Cited by
-
Emerging Polymer Materials in Trackable Endovascular Embolization and Cell Delivery: From Hype to Hope.Biomimetics (Basel). 2022 Jun 10;7(2):77. doi: 10.3390/biomimetics7020077. Biomimetics (Basel). 2022. PMID: 35735593 Free PMC article. Review.
-
Initial Experience of Drug-Eluting Bead-Transcatheter Arterial Chemoembolization After Lipiodol-Based Transcatheter Arterial Chemoembolization Failure for Patients with Advanced Hepatocellular Carcinoma.Cancer Manag Res. 2021 Oct 19;13:7973-7980. doi: 10.2147/CMAR.S332571. eCollection 2021. Cancer Manag Res. 2021. PMID: 34703317 Free PMC article.
-
Hepatocellular carcinoma treatment: hurdles, advances and prospects.Hepat Oncol. 2018 Sep 28;5(2):HEP08. doi: 10.2217/hep-2018-0002. eCollection 2018 Apr. Hepat Oncol. 2018. PMID: 31293776 Free PMC article. Review.
-
A Retrospective Cohort Analysis of Transarterial Chemoembolization for Hepatocellular Cancer at a Tertiary Center in Switzerland.J Clin Med. 2024 Jun 2;13(11):3279. doi: 10.3390/jcm13113279. J Clin Med. 2024. PMID: 38892990 Free PMC article.
-
Efficacy of transcatheter arterial chemoembolization using pirarubicin-loaded microspheres combined with lobaplatin for primary liver cancer.World J Clin Cases. 2022 Sep 26;10(27):9650-9656. doi: 10.12998/wjcc.v10.i27.9650. World J Clin Cases. 2022. PMID: 36186198 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

