NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice
- PMID: 28167322
- PMCID: PMC6536116
- DOI: 10.1016/j.jhep.2017.01.022
NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice
Abstract
Background & aims: NOD-like receptor protein 3 (NLRP3) inflammasome activation occurs in Non-alcoholic fatty liver disease (NAFLD). We used the first small molecule NLRP3 inhibitor, MCC950, to test whether inflammasome blockade alters inflammatory recruitment and liver fibrosis in two murine models of steatohepatitis.
Methods: We fed foz/foz and wild-type mice an atherogenic diet for 16weeks, gavaged MCC950 or vehicle until 24weeks, then determined NAFLD phenotype. In mice fed an methionine/choline deficient (MCD) diet, we gavaged MCC950 or vehicle for 6weeks and determined the effects on liver fibrosis.
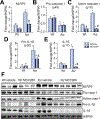
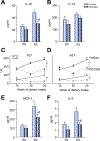
Results: In vehicle-treated foz/foz mice, hepatic expression of NLRP3, pro-IL-1β, active caspase-1 and IL-1β increased at 24weeks, in association with cholesterol crystal formation and NASH pathology; plasma IL-1β, IL-6, MCP-1, ALT/AST all increased. MCC950 treatment normalized hepatic caspase 1 and IL-1β expression, plasma IL-1β, MCP-1 and IL-6, lowered ALT/AST, and reduced the severity of liver inflammation including designation as NASH pathology, and liver fibrosis. In vitro, cholesterol crystals activated Kupffer cells and macrophages to release IL-1β; MCC950 abolished this, and the associated neutrophil migration. MCD diet-fed mice developed fibrotic steatohepatitis; MCC950 suppressed the increase in hepatic caspase 1 and IL-1β, lowered numbers of macrophages and neutrophils in the liver, and improved liver fibrosis.
Conclusion: MCC950, an NLRP3 selective inhibitor, improved NAFLD pathology and fibrosis in obese diabetic mice. This is potentially attributable to the blockade of cholesterol crystal-mediated NLRP3 activation in myeloid cells. MCC950 reduced liver fibrosis in MCD-fed mice. Targeting NLRP3 is a logical direction in pharmacotherapy of NASH.
Lay summary: Fatty liver disease caused by being overweight with diabetes and a high risk of heart attack, termed non-alcoholic steatohepatitis (NASH), is the most common serious liver disease with no current treatment. There could be several causes of inflammation in NASH, but activation of a protein scaffold within cells termed the inflammasome (NLRP3) has been suggested to play a role. Here we show that cholesterol crystals could be one pathway to activate the inflammasome in NASH. We used a drug called MCC950, which has already been shown to block NLRP3 activation, in an attempt to reduce liver injury in NASH. This drug partly reversed liver inflammation, particularly in obese diabetic mice that most closely resembles the human context of NASH. In addition, such dampening of liver inflammation in NASH achieved with MCC950 partly reversed liver scarring, the process that links NASH to the development of cirrhosis.
Keywords: Cholesterol crystals; Diet, atherogenic; Fibrosis; Hepatocytes; Inflammasomes; Interleukin-1β; Kupffer cells; Methionine; NAFLD; NLR proteins; NLRP3.
Copyright © 2017 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
ARM, AW, MMY, CDJ, DVR, FH, NCT, CS, GNI, SLM, AEF and GCF have nothing to disclose. AABR, KS, and MAC are co-inventors on a patent application filed by The University of Queensland describing novel small molecules inhibitors of the NLRP3 inflammasome. However, none of these new molecules are described in the present work, which is limited to the public domain, non-patented compound MCC950. Hence there is no direct conflict of interest in this work describing the role of MCC950 in NASH, but the commercial association with other inhibitors of the inflammasome described in said patent application are declared.
Figures
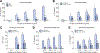



Comment in
-
NAFLD: A critical role for the NLRP3 inflammasome in NASH.Nat Rev Gastroenterol Hepatol. 2017 Apr;14(4):197. doi: 10.1038/nrgastro.2017.21. Epub 2017 Feb 22. Nat Rev Gastroenterol Hepatol. 2017. PMID: 28223701 No abstract available.
References
-
- Ahmed A, Wong RJ, Harrison SA. Nonalcoholic fatty liver disease review: Diagnosis, treatment, and outcomes. Clin Gastroenterol Hepatol 2015;13: 2062–2070. - PubMed
-
- Farrell GC, McCullough AJ, Day CP, editors. Non-alcoholic fatty liver disease: A practical guide. Chichester: Wiley-Blackwell; 2013.
-
- Torres DM, Harrison SA. Nonalcoholic fatty liver disease: Fibrosis portends a worse prognosis. Hepatology 2015;61: 1462–1464. - PubMed
-
- Cusi K Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012;142: 711–725. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

