Adaptation of a Genetic Screen Reveals an Inhibitor for Mitochondrial Protein Import Component Tim44
- PMID: 28167535
- PMCID: PMC5392686
- DOI: 10.1074/jbc.M116.770131
Adaptation of a Genetic Screen Reveals an Inhibitor for Mitochondrial Protein Import Component Tim44
Abstract
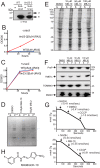
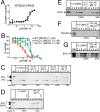
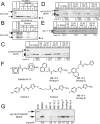
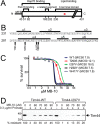
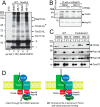
Diverse protein import pathways into mitochondria use translocons on the outer membrane (TOM) and inner membrane (TIM). We adapted a genetic screen, based on Ura3 mistargeting from mitochondria to the cytosol, to identify small molecules that attenuated protein import. Small molecule mitochondrial import blockers of the Carla Koehler laboratory (MB)-10 inhibited import of substrates that require the TIM23 translocon. Mutational analysis coupled with molecular docking and molecular dynamics modeling revealed that MB-10 binds to a specific pocket in the C-terminal domain of Tim44 of the protein-associated motor (PAM) complex. This region was proposed to anchor Tim44 to the membrane, but biochemical studies with MB-10 show that this region is required for binding to the translocating precursor and binding to mtHsp70 in low ATP conditions. This study also supports a direct role for the PAM complex in the import of substrates that are laterally sorted to the inner membrane, as well as the mitochondrial matrix. Thus, MB-10 is the first small molecule modulator to attenuate PAM complex activity, likely through binding to the C-terminal region of Tim44.
Keywords: chemical biology; mitochondria; mitochondrial transport; protein translocation; small molecule.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Mitochondrial protein import motor: differential role of Tim44 in the recruitment of Pam17 and J-complex to the presequence translocase.Mol Biol Cell. 2008 Jun;19(6):2642-9. doi: 10.1091/mbc.e07-12-1226. Epub 2008 Apr 9. Mol Biol Cell. 2008. PMID: 18400944 Free PMC article.
-
Residues of Tim44 involved in both association with the translocon of the inner mitochondrial membrane and regulation of mitochondrial Hsp70 tethering.Mol Cell Biol. 2008 Jul;28(13):4424-33. doi: 10.1128/MCB.00007-08. Epub 2008 Apr 21. Mol Cell Biol. 2008. PMID: 18426906 Free PMC article.
-
Architecture of the TIM23 inner mitochondrial translocon and interactions with the matrix import motor.J Biol Chem. 2014 Oct 10;289(41):28689-96. doi: 10.1074/jbc.M114.588152. Epub 2014 Aug 25. J Biol Chem. 2014. PMID: 25157107 Free PMC article.
-
On the mechanism of preprotein import by the mitochondrial presequence translocase.Biochim Biophys Acta. 2010 Jun;1803(6):732-9. doi: 10.1016/j.bbamcr.2010.01.013. Epub 2010 Jan 25. Biochim Biophys Acta. 2010. PMID: 20100523 Review.
-
Mitochondrial preprotein translocases as dynamic molecular machines.FEMS Yeast Res. 2006 Sep;6(6):849-61. doi: 10.1111/j.1567-1364.2006.00134.x. FEMS Yeast Res. 2006. PMID: 16911507 Review.
Cited by
-
TIMM44 is a potential therapeutic target of human glioma.Theranostics. 2022 Oct 31;12(17):7586-7602. doi: 10.7150/thno.78616. eCollection 2022. Theranostics. 2022. PMID: 36438483 Free PMC article.
-
The Interaction and Effect of a Small MitoBlock Library as Inhibitor of ALR Protein-Protein Interaction Pathway.Int J Mol Sci. 2024 Jan 18;25(2):1174. doi: 10.3390/ijms25021174. Int J Mol Sci. 2024. PMID: 38256258 Free PMC article.
-
Targeting Mitochondrial Protein Expression as a Future Approach for Cancer Therapy.Front Oncol. 2021 Nov 23;11:797265. doi: 10.3389/fonc.2021.797265. eCollection 2021. Front Oncol. 2021. PMID: 34888254 Free PMC article. Review.
-
MitoPlex: A targeted multiple reaction monitoring assay for quantification of a curated set of mitochondrial proteins.J Mol Cell Cardiol. 2020 May;142:1-13. doi: 10.1016/j.yjmcc.2020.03.011. Epub 2020 Mar 29. J Mol Cell Cardiol. 2020. PMID: 32234390 Free PMC article.
-
The mitochondrial protein TIMM44 is required for angiogenesis in vitro and in vivo.Cell Death Dis. 2023 May 5;14(5):307. doi: 10.1038/s41419-023-05826-9. Cell Death Dis. 2023. PMID: 37147302 Free PMC article.
References
-
- Neupert W., and Herrmann J. M. (2007) Translocation of proteins into mitochondria. Annu. Rev. Biochem. 76, 723–749 - PubMed
-
- Alder N. N., Jensen R. E., and Johnson A. E. (2008) Fluorescence mapping of mitochondrial TIM23 complex reveals a water-facing, substrate-interacting helix surface. Cell 134, 439–450 - PubMed
-
- Meinecke M., Wagner R., Kovermann P., Guiard B., Mick D. U., Hutu D. P., Voos W., Truscott K. N., Chacinska A., Pfanner N., and Rehling P. (2006) Tim50 maintains the permeability barrier of the mitochondrial inner membrane. Science 312, 1523–1526 - PubMed
-
- van der Laan M., Meinecke M., Dudek J., Hutu D. P., Lind M., Perschil I., Guiard B., Wagner R., Pfanner N., and Rehling P. (2007) Motor-free mitochondrial presequence translocase drives membrane integration of preproteins. Nat. Cell Biol. 9, 1152–1159 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

