Enterocin AS-48 as Evidence for the Use of Bacteriocins as New Leishmanicidal Agents
- PMID: 28167557
- PMCID: PMC5365675
- DOI: 10.1128/AAC.02288-16
Enterocin AS-48 as Evidence for the Use of Bacteriocins as New Leishmanicidal Agents
Abstract
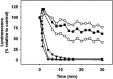
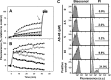
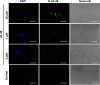
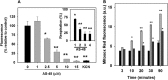
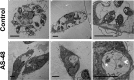
We report the feasibility of enterocin AS-48, a circular cationic peptide produced by Enterococcus faecalis, as a new leishmanicidal agent. AS-48 is lethal to Leishmania promastigotes as well as to axenic and intracellular amastigotes at low micromolar concentrations, with scarce cytotoxicity to macrophages. AS-48 induced a fast bioenergetic collapse of L. donovani promastigotes but only a partial permeation of their plasma membrane with limited entrance of vital dyes, even at concentrations beyond its full lethality. Fluoresceinated AS-48 was visualized inside parasites by confocal microscopy and seen to cause mitochondrial depolarization and reactive oxygen species production. Altogether, AS-48 appeared to have a mixed leishmanicidal mechanism that includes both plasma membrane permeabilization and additional intracellular targets, with mitochondrial dysfunctionality being of special relevance. This complex leishmanicidal mechanism of AS-48 persisted even for the killing of intracellular amastigotes, as evidenced by transmission electron microscopy. We demonstrated the potentiality of AS-48 as a new and safe leishmanicidal agent, expanding the growing repertoire of eukaryotic targets for bacteriocins, and our results provide a proof of mechanism for the search of new leishmanicidal bacteriocins, whose diversity constitutes an almost endless source for new structures at moderate production cost and whose safe use on food preservation is well established.
Keywords: antimicrobial peptide; bioenergetics; enterocin AS-48; intracellular parasite.
Copyright © 2017 American Society for Microbiology.
Figures
Similar articles
-
Fungus-elicited metabolites from plants as an enriched source for new leishmanicidal agents: antifungal phenyl-phenalenone phytoalexins from the banana plant (Musa acuminata) target mitochondria of Leishmania donovani promastigotes.Antimicrob Agents Chemother. 2004 May;48(5):1534-40. doi: 10.1128/AAC.48.5.1534-1540.2004. Antimicrob Agents Chemother. 2004. PMID: 15105102 Free PMC article.
-
15d-Prostaglandin J2 induced reactive oxygen species-mediated apoptosis during experimental visceral leishmaniasis.J Mol Med (Berl). 2016 Jun;94(6):695-710. doi: 10.1007/s00109-016-1384-5. Epub 2016 Feb 1. J Mol Med (Berl). 2016. PMID: 26830627
-
Apoptosis-like cell death in Leishmania donovani treated with KalsomeTM10, a new liposomal amphotericin B.PLoS One. 2017 Feb 7;12(2):e0171306. doi: 10.1371/journal.pone.0171306. eCollection 2017. PLoS One. 2017. PMID: 28170432 Free PMC article.
-
Human antimicrobial peptide histatin 5 is a cell-penetrating peptide targeting mitochondrial ATP synthesis in Leishmania.FASEB J. 2008 Jun;22(6):1817-28. doi: 10.1096/fj.07-096081. Epub 2008 Jan 29. FASEB J. 2008. PMID: 18230684
-
Stearylamine-bearing cationic liposomes kill Leishmania parasites through surface exposed negatively charged phosphatidylserine.J Antimicrob Chemother. 2008 Jan;61(1):103-10. doi: 10.1093/jac/dkm396. Epub 2007 Oct 26. J Antimicrob Chemother. 2008. PMID: 17965031
Cited by
-
Library of Selenocyanate and Diselenide Derivatives as In Vivo Antichagasic Compounds Targeting Trypanosoma cruzi Mitochondrion.Pharmaceuticals (Basel). 2021 May 1;14(5):419. doi: 10.3390/ph14050419. Pharmaceuticals (Basel). 2021. PMID: 34062791 Free PMC article.
-
Advances in the preclinical characterization of the antimicrobial peptide AS-48.Front Microbiol. 2023 Feb 2;14:1110360. doi: 10.3389/fmicb.2023.1110360. eCollection 2023. Front Microbiol. 2023. PMID: 36819031 Free PMC article. Review.
-
The Networked Interaction between Probiotics and Intestine in Health and Disease: A Promising Success Story.Microorganisms. 2024 Jan 18;12(1):194. doi: 10.3390/microorganisms12010194. Microorganisms. 2024. PMID: 38258020 Free PMC article. Review.
-
The Genus Enterococcus: Between Probiotic Potential and Safety Concerns-An Update.Front Microbiol. 2018 Aug 3;9:1791. doi: 10.3389/fmicb.2018.01791. eCollection 2018. Front Microbiol. 2018. PMID: 30123208 Free PMC article. Review.
-
Preclinical studies of toxicity and safety of the AS-48 bacteriocin.J Adv Res. 2019 Jul 4;20:129-139. doi: 10.1016/j.jare.2019.06.003. eCollection 2019 Nov. J Adv Res. 2019. PMID: 31360546 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

