The familial dysautonomia disease gene IKBKAP is required in the developing and adult mouse central nervous system
- PMID: 28167615
- PMCID: PMC5451171
- DOI: 10.1242/dmm.028258
The familial dysautonomia disease gene IKBKAP is required in the developing and adult mouse central nervous system
Abstract
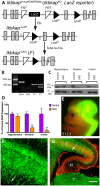
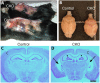
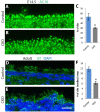
Hereditary sensory and autonomic neuropathies (HSANs) are a genetically and clinically diverse group of disorders defined by peripheral nervous system (PNS) dysfunction. HSAN type III, known as familial dysautonomia (FD), results from a single base mutation in the gene IKBKAP that encodes a scaffolding unit (ELP1) for a multi-subunit complex known as Elongator. Since mutations in other Elongator subunits (ELP2 to ELP4) are associated with central nervous system (CNS) disorders, the goal of this study was to investigate a potential requirement for Ikbkap in the CNS of mice. The sensory and autonomic pathophysiology of FD is fatal, with the majority of patients dying by age 40. While signs and pathology of FD have been noted in the CNS, the clinical and research focus has been on the sensory and autonomic dysfunction, and no genetic model studies have investigated the requirement for Ikbkap in the CNS. Here, we report, using a novel mouse line in which Ikbkap is deleted solely in the nervous system, that not only is Ikbkap widely expressed in the embryonic and adult CNS, but its deletion perturbs both the development of cortical neurons and their survival in adulthood. Primary cilia in embryonic cortical apical progenitors and motile cilia in adult ependymal cells are reduced in number and disorganized. Furthermore, we report that, in the adult CNS, both autonomic and non-autonomic neuronal populations require Ikbkap for survival, including spinal motor and cortical neurons. In addition, the mice developed kyphoscoliosis, an FD hallmark, indicating its neuropathic etiology. Ultimately, these perturbations manifest in a developmental and progressive neurodegenerative condition that includes impairments in learning and memory. Collectively, these data reveal an essential function for Ikbkap that extends beyond the peripheral nervous system to CNS development and function. With the identification of discrete CNS cell types and structures that depend on Ikbkap, novel strategies to thwart the progressive demise of CNS neurons in FD can be developed.
Keywords: Elongator; Familial dysautonomia; Hereditary sensory and autonomic neuropathy.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

