ORAI1 Activates Proliferation of Lymphatic Endothelial Cells in Response to Laminar Flow Through Krüppel-Like Factors 2 and 4
- PMID: 28167653
- PMCID: PMC6300148
- DOI: 10.1161/CIRCRESAHA.116.309548
ORAI1 Activates Proliferation of Lymphatic Endothelial Cells in Response to Laminar Flow Through Krüppel-Like Factors 2 and 4
Erratum in
-
Correction to: ORAI1 Activates Proliferation of Lymphatic Endothelial Cells in Response to Laminar Flow Through Krüppel-Like Factors 2 and 4.Circ Res. 2024 May 24;134(11):e176. doi: 10.1161/RES.0000000000000673. Epub 2024 May 23. Circ Res. 2024. PMID: 38781305 No abstract available.
Abstract
Rationale: Lymphatic vessels function to drain interstitial fluid from a variety of tissues. Although shear stress generated by fluid flow is known to trigger lymphatic expansion and remodeling, the molecular basis underlying flow-induced lymphatic growth is unknown.
Objective: We aimed to gain a better understanding of the mechanism by which laminar shear stress activates lymphatic proliferation.
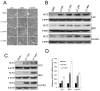
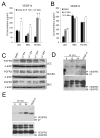
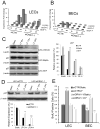
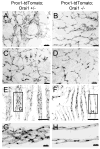
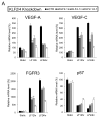
Methods and results: Primary endothelial cells from dermal blood and lymphatic vessels (blood vascular endothelial cells and lymphatic endothelial cells [LECs]) were exposed to low-rate steady laminar flow. Shear stress-induced molecular and cellular responses were defined and verified using various mutant mouse models. Steady laminar flow induced the classic shear stress responses commonly in blood vascular endothelial cells and LECs. Surprisingly, however, only LECs showed enhanced cell proliferation by regulating the vascular endothelial growth factor (VEGF)-A, VEGF-C, FGFR3, and p57/CDKN1C genes. As an early signal mediator, ORAI1, a pore subunit of the calcium release-activated calcium channel, was identified to induce the shear stress phenotypes and cell proliferation in LECs responding to the fluid flow. Mechanistically, ORAI1 induced upregulation of Krüppel-like factor (KLF)-2 and KLF4 in the flow-activated LECs, and the 2 KLF proteins cooperate to regulate VEGF-A, VEGF-C, FGFR3, and p57 by binding to the regulatory regions of the genes. Consistently, freshly isolated LECs from Orai1 knockout embryos displayed reduced expression of KLF2, KLF4, VEGF-A, VEGF-C, and FGFR3 and elevated expression of p57. Accordingly, mouse embryos deficient in Orai1, Klf2, or Klf4 showed a significantly reduced lymphatic density and impaired lymphatic development.
Conclusions: Our study identified a molecular mechanism for laminar flow-activated LEC proliferation.
Keywords: calcium channel; capillary; cell proliferation; lymphatic vessels; vascular endothelial growth factor A.
© 2017 American Heart Association, Inc.
Conflict of interest statement
The authors declare no conflict of interest with this study.
Figures








Comment in
-
ORAI1 Controls the Unique Adjustment of Lymphatics to Fluid Flow.Circ Res. 2017 Apr 28;120(9):1373-1375. doi: 10.1161/CIRCRESAHA.117.310917. Circ Res. 2017. PMID: 28450355 No abstract available.
References
-
- Sabine A, Agalarov Y, Maby-El Hajjami H, Jaquet M, Hagerling R, Pollmann C, Bebber D, Pfenniger A, Miura N, Dormond O, Calmes JM, Adams RH, Makinen T, Kiefer F, Kwak BR, Petrova TV. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell. 2012;22:430–445. - PubMed
-
- Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981;103:177–185. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

