Mucosal Antibodies to the C Terminus of Toxin A Prevent Colonization of Clostridium difficile
- PMID: 28167669
- PMCID: PMC5364299
- DOI: 10.1128/IAI.01060-16
Mucosal Antibodies to the C Terminus of Toxin A Prevent Colonization of Clostridium difficile
Erratum in
-
Erratum for Hong et al., "Mucosal Antibodies to the C Terminus of Toxin A Prevent Colonization of Clostridium difficile".Infect Immun. 2017 May 23;85(6):e00279-17. doi: 10.1128/IAI.00279-17. Print 2017 Jun. Infect Immun. 2017. PMID: 28536258 Free PMC article. No abstract available.
Abstract
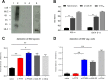
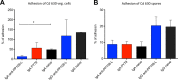
Mucosal immunity is considered important for protection against Clostridium difficile infection (CDI). We show that in hamsters immunized with Bacillus subtilis spores expressing a carboxy-terminal segment (TcdA26-39) of C. difficile toxin A, no colonization occurs in protected animals when challenged with C. difficile strain 630. In contrast, animals immunized with toxoids showed no protection and remained fully colonized. Along with neutralizing toxins, antibodies to TcdA26-39 (but not to toxoids), whether raised to the recombinant protein or to TcdA26-39 expressed on the B. subtilis spore surface, cross-react with a number of seemingly unrelated proteins expressed on the vegetative cell surface or spore coat of C. difficile These include two dehydrogenases, AdhE1 and LdhA, as well as the CdeC protein that is present on the spore. Anti-TcdA26-39 mucosal antibodies obtained following immunization with recombinant B. subtilis spores were able to reduce the adhesion of C. difficile to mucus-producing intestinal cells. This cross-reaction is intriguing yet important since it illustrates the importance of mucosal immunity for complete protection against CDI.
Keywords: Clostridium difficile; colonization; immune exclusion; mucosal immunity; oral vaccines.
Copyright © 2017 American Society for Microbiology.
Figures





Similar articles
-
Immunization with Bacillus spores expressing toxin A peptide repeats protects against infection with Clostridium difficile strains producing toxins A and B.Infect Immun. 2011 Jun;79(6):2295-302. doi: 10.1128/IAI.00130-11. Epub 2011 Apr 11. Infect Immun. 2011. PMID: 21482682 Free PMC article.
-
Oral Immunization with Nontoxigenic Clostridium difficile Strains Expressing Chimeric Fragments of TcdA and TcdB Elicits Protective Immunity against C. difficile Infection in Both Mice and Hamsters.Infect Immun. 2018 Oct 25;86(11):e00489-18. doi: 10.1128/IAI.00489-18. Print 2018 Nov. Infect Immun. 2018. PMID: 30150259 Free PMC article.
-
Systemic antibody responses induced by a two-component Clostridium difficile toxoid vaccine protect against C. difficile-associated disease in hamsters.J Med Microbiol. 2013 Sep;62(Pt 9):1394-1404. doi: 10.1099/jmm.0.056796-0. Epub 2013 Mar 21. J Med Microbiol. 2013. PMID: 23518659
-
Against Clostridioides difficile Infection: An Update on Vaccine Development.Toxins (Basel). 2025 May 1;17(5):222. doi: 10.3390/toxins17050222. Toxins (Basel). 2025. PMID: 40423305 Free PMC article. Review.
-
Enterotoxic Clostridia: Clostridioides difficile Infections.Microbiol Spectr. 2019 May;7(3):10.1128/microbiolspec.gpp3-0015-2018. doi: 10.1128/microbiolspec.GPP3-0015-2018. Microbiol Spectr. 2019. PMID: 31124432 Free PMC article. Review.
Cited by
-
Mechanisms and Applications of Bacterial Sporulation and Germination in the Intestine.Int J Mol Sci. 2022 Mar 21;23(6):3405. doi: 10.3390/ijms23063405. Int J Mol Sci. 2022. PMID: 35328823 Free PMC article. Review.
-
Recent advances in the treatment of C. difficile using biotherapeutic agents.Infect Drug Resist. 2019 Jun 10;12:1597-1615. doi: 10.2147/IDR.S207572. eCollection 2019. Infect Drug Resist. 2019. PMID: 31354309 Free PMC article.
-
Bacterial Spore-Based Delivery System: 20 Years of a Versatile Approach for Innovative Vaccines.Biomolecules. 2023 Jun 6;13(6):947. doi: 10.3390/biom13060947. Biomolecules. 2023. PMID: 37371527 Free PMC article. Review.
-
Bioengineered Probiotics for Clostridioides difficile Infection: An Overview of the Challenges and Potential for This New Treatment Approach.Probiotics Antimicrob Proteins. 2025 Apr;17(2):763-780. doi: 10.1007/s12602-024-10398-x. Epub 2024 Nov 12. Probiotics Antimicrob Proteins. 2025. PMID: 39531149 Review.
-
Development of an Effective Nontoxigenic Clostridioides difficile-Based Oral Vaccine against C. difficile Infection.Microbiol Spectr. 2022 Jun 29;10(3):e0026322. doi: 10.1128/spectrum.00263-22. Epub 2022 May 18. Microbiol Spectr. 2022. PMID: 35583336 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

