Low Hesperian PCO2 constrained from in situ mineralogical analysis at Gale Crater, Mars
- PMID: 28167765
- PMCID: PMC5338541
- DOI: 10.1073/pnas.1616649114
Low Hesperian PCO2 constrained from in situ mineralogical analysis at Gale Crater, Mars
Abstract
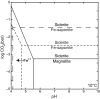
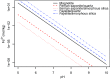
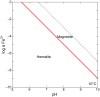
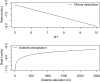
Carbon dioxide is an essential atmospheric component in martian climate models that attempt to reconcile a faint young sun with planetwide evidence of liquid water in the Noachian and Early Hesperian. In this study, we use mineral and contextual sedimentary environmental data measured by the Mars Science Laboratory (MSL) Rover Curiosity to estimate the atmospheric partial pressure of CO2 (PCO2) coinciding with a long-lived lake system in Gale Crater at ∼3.5 Ga. A reaction-transport model that simulates mineralogy observed within the Sheepbed member at Yellowknife Bay (YKB), by coupling mineral equilibria with carbonate precipitation kinetics and rates of sedimentation, indicates atmospheric PCO2 levels in the 10s mbar range. At such low PCO2 levels, existing climate models are unable to warm Hesperian Mars anywhere near the freezing point of water, and other gases are required to raise atmospheric pressure to prevent lake waters from being lost to the atmosphere. Thus, either lacustrine features of Gale formed in a cold environment by a mechanism yet to be determined, or the climate models still lack an essential component that would serve to elevate surface temperatures, at least locally, on Hesperian Mars. Our results also impose restrictions on the potential role of atmospheric CO2 in inferred warmer conditions and valley network formation of the late Noachian.
Keywords: Gale Crater; Hesperian Mars; Mars Science Laboratory; carbon dioxide; martian atmosphere.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

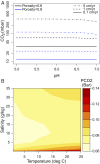
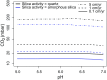
References
-
- Carr MF. Water on Mars. Oxford Univ Press; New York: 1996.
-
- Di Achille G, Hynek BM. Ancient ocean on Mars supported by global distribution of deltas and valleys. Nat Geosci. 2010;3:459–463.
-
- Bibring J-P, et al. Global mineralogical and aqueous mars history derived from OMEGA/Mars Express data. Science. 2006;312(5772):400–404. - PubMed
-
- Murchie SL, et al. 2009. A synthesis of Martian aqueous mineralogy after 1 Mars year of observations from the Mars Reconnaissance Orbiter. J Geophys Res 114(E2): 10.1029/2009JE003342.
-
- Niles PB, et al. Geochemistry of carbonates on Mars: Implications for climate history and nature of aqueous environments. Space Sci Rev. 2012;174(1):301–328.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

