Selection maintains signaling function of a highly diverged intrinsically disordered region
- PMID: 28167781
- PMCID: PMC5338452
- DOI: 10.1073/pnas.1614787114
Selection maintains signaling function of a highly diverged intrinsically disordered region
Abstract
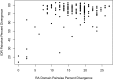
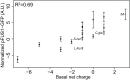
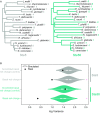
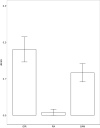
Intrinsically disordered regions (IDRs) are characterized by their lack of stable secondary or tertiary structure and comprise a large part of the eukaryotic proteome. Although these regions play a variety of signaling and regulatory roles, they appear to be rapidly evolving at the primary sequence level. To understand the functional implications of this rapid evolution, we focused on a highly diverged IDR in Saccharomyces cerevisiae that is involved in regulating multiple conserved MAPK pathways. We hypothesized that under stabilizing selection, the functional output of orthologous IDRs could be maintained, such that diverse genotypes could lead to similar function and fitness. Consistent with the stabilizing selection hypothesis, we find that diverged, orthologous IDRs can mostly recapitulate wild-type function and fitness in S. cerevisiae We also find that the electrostatic charge of the IDR is correlated with signaling output and, using phylogenetic comparative methods, find evidence for selection maintaining this quantitative molecular trait despite underlying genotypic divergence.
Keywords: evolution; intrinsically disordered; phylogenetic comparative method; quantitative trait; stabilizing selection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT. Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol. 2004;337(3):635–645. - PubMed
-
- Peng Z, Mizianty MJ, Kurgan L. Genome-scale prediction of proteins with long intrinsically disordered regions. Proteins. 2014;82(1):145–158. - PubMed
-
- Vavouri T, Semple JI, Garcia-Verdugo R, Lehner B. Intrinsic protein disorder and interaction promiscuity are widely associated with dosage sensitivity. Cell. 2009;138(1):198–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

