Enhancement of Anti-Inflammatory and Osteogenic Abilities of Mesenchymal Stem Cells via Cell-to-Cell Adhesion to Periodontal Ligament-Derived Fibroblasts
- PMID: 28167967
- PMCID: PMC5266859
- DOI: 10.1155/2017/3296498
Enhancement of Anti-Inflammatory and Osteogenic Abilities of Mesenchymal Stem Cells via Cell-to-Cell Adhesion to Periodontal Ligament-Derived Fibroblasts
Abstract
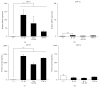
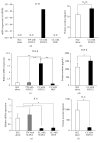
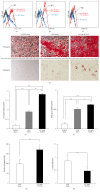
Mesenchymal stem cells (MSCs) are involved in anti-inflammatory events and tissue repair; these functions are activated by their migration or homing to inflammatory tissues in response to various chemokines. However, the mechanism by which MSCs interact with other cell types in inflammatory tissue remains unclear. We investigated the role of periodontal ligament fibroblasts (PDL-Fs) in regulating the anti-inflammatory and osteogenic abilities of bone marrow-derived- (BM-) MSCs. The expression of monocyte chemotactic protein- (MCP-)1 was significantly enhanced by stimulation of PDL-Fs with inflammatory cytokines. MCP-1 induced the migratory ability of BM-MSCs but not PDL-Fs. Expression levels of anti-inflammatory and inflammatory cytokines were increased and decreased, respectively, by direct-contact coculture between MSCs and PDL-Fs. In addition, the direct-contact coculture enhanced the expression of MSC markers that play important roles in the self-renewal and maintenance of multipotency of MSCs, which in turn induced the osteogenic ability of the cells. These results suggest that MCP-1 induces the migration and homing of BM-MSCs into the PDL inflammatory tissue. The subsequent adherence of MSCs to PDL-Fs plays an immunomodulatory role to terminate inflammation during wound healing and upregulates the expression stem cell markers to enhance the stemness of MSCs, thereby facilitating bone formation in damaged PDL tissue.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Friedenstein A. J., Chailakhjan R. K., Lalykina K. S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell and Tissue Kinetics. 1970;3(4):393–403. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

