Role of the IL-33-ST2 axis in sepsis
- PMID: 28168040
- PMCID: PMC5288881
- DOI: 10.1186/s40779-017-0115-8
Role of the IL-33-ST2 axis in sepsis
Abstract
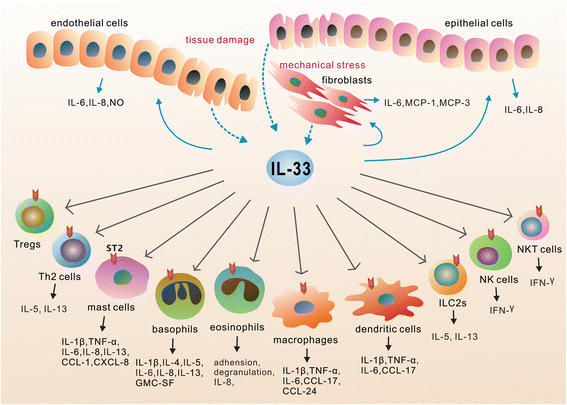
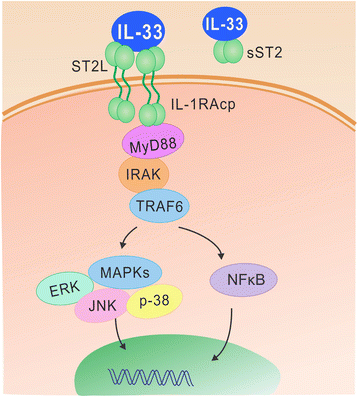
Sepsis remains a major clinical problem with high morbidity and mortality. As new inflammatory mediators are characterized, it is important to understand their roles in sepsis. Interleukin 33 (IL-33) is a recently described member of the IL-1 family that is widely expressed in cells of barrier tissues. Upon tissue damage, IL-33 is released as an alarmin and activates various types of cells of both the innate and adaptive immune system through binding to the ST2/IL-1 receptor accessory protein complex. IL-33 has apparent pleiotropic functions in many disease models, with its actions strongly shaped by the local microenvironment. Recent studies have established a role for the IL-33-ST2 axis in the initiation and perpetuation of inflammation during endotoxemia, but its roles in sepsis appear to be organism and model dependent. In this review, we focus on the recent advances in understanding the role of the IL-33/ST2 axis in sepsis.
Keywords: ST2; Sepsis, Interleukin-33.
Figures
Similar articles
-
Crucial and diverse role of the interleukin-33/ST2 axis in infectious diseases.Infect Immun. 2015 May;83(5):1738-48. doi: 10.1128/IAI.02908-14. Epub 2015 Feb 23. Infect Immun. 2015. PMID: 25712928 Free PMC article. Review.
-
Emerging Roles of IL-33/ST2 Axis in Renal Diseases.Int J Mol Sci. 2017 Apr 7;18(4):783. doi: 10.3390/ijms18040783. Int J Mol Sci. 2017. PMID: 28387719 Free PMC article. Review.
-
IL-33/IL-31 Axis: A Potential Inflammatory Pathway.Mediators Inflamm. 2018 Mar 11;2018:3858032. doi: 10.1155/2018/3858032. eCollection 2018. Mediators Inflamm. 2018. PMID: 29713240 Free PMC article. Review.
-
Role of IL-33-ST2 pathway in regulating inflammation: current evidence and future perspectives.J Transl Med. 2023 Dec 11;21(1):902. doi: 10.1186/s12967-023-04782-4. J Transl Med. 2023. PMID: 38082335 Free PMC article. Review.
-
The IL-33/ST2 axis: Role in health and disease.Cytokine Growth Factor Rev. 2015 Dec;26(6):615-23. doi: 10.1016/j.cytogfr.2015.07.017. Epub 2015 Jul 28. Cytokine Growth Factor Rev. 2015. PMID: 26271893 Review.
Cited by
-
Celastrol mitigates inflammation in sepsis by inhibiting the PKM2-dependent Warburg effect.Mil Med Res. 2022 May 20;9(1):22. doi: 10.1186/s40779-022-00381-4. Mil Med Res. 2022. PMID: 35596191 Free PMC article.
-
IL-33 and Soluble ST2 Are Associated With Recurrent Spontaneous Abortion in Early Pregnancy.Front Physiol. 2022 Jan 13;12:789829. doi: 10.3389/fphys.2021.789829. eCollection 2021. Front Physiol. 2022. PMID: 35095557 Free PMC article.
-
NLRP3 inhibition improves maternal hypertension, inflammation, and vascular dysfunction in response to placental ischemia.Am J Physiol Regul Integr Comp Physiol. 2023 Apr 1;324(4):R556-R567. doi: 10.1152/ajpregu.00192.2022. Epub 2023 Feb 27. Am J Physiol Regul Integr Comp Physiol. 2023. PMID: 36847598 Free PMC article.
-
Are histones real pathogenic agents in sepsis?Nat Rev Immunol. 2018 Feb;18(2):148. doi: 10.1038/nri.2017.157. Epub 2017 Dec 27. Nat Rev Immunol. 2018. PMID: 29279614 No abstract available.
-
Anti-DAMP therapies for acute inflammation.Front Immunol. 2025 May 8;16:1579954. doi: 10.3389/fimmu.2025.1579954. eCollection 2025. Front Immunol. 2025. PMID: 40406124 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

