Diversity of Cnidarian Muscles: Function, Anatomy, Development and Regeneration
- PMID: 28168188
- PMCID: PMC5253434
- DOI: 10.3389/fcell.2016.00157
Diversity of Cnidarian Muscles: Function, Anatomy, Development and Regeneration
Abstract
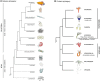
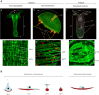
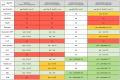
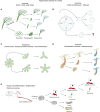
The ability to perform muscle contractions is one of the most important and distinctive features of eumetazoans. As the sister group to bilaterians, cnidarians (sea anemones, corals, jellyfish, and hydroids) hold an informative phylogenetic position for understanding muscle evolution. Here, we review current knowledge on muscle function, diversity, development, regeneration and evolution in cnidarians. Cnidarian muscles are involved in various activities, such as feeding, escape, locomotion and defense, in close association with the nervous system. This variety is reflected in the large diversity of muscle organizations found in Cnidaria. Smooth epithelial muscle is thought to be the most common type, and is inferred to be the ancestral muscle type for Cnidaria, while striated muscle fibers and non-epithelial myocytes would have been convergently acquired within Cnidaria. Current knowledge of cnidarian muscle development and its regeneration is limited. While orthologs of myogenic regulatory factors such as MyoD have yet to be found in cnidarian genomes, striated muscle formation potentially involves well-conserved myogenic genes, such as twist and mef2. Although satellite cells have yet to be identified in cnidarians, muscle plasticity (e.g., de- and re-differentiation, fiber repolarization) in a regenerative context and its potential role during regeneration has started to be addressed in a few cnidarian systems. The development of novel tools to study those organisms has created new opportunities to investigate in depth the development and regeneration of cnidarian muscle cells and how they contribute to the regenerative process.
Keywords: cnidaria; development; epitheliomuscular cells; evolution; muscle; myoepithelial cells; regeneration.
Figures


References
-
- Aerne B., Gröger H., Schuchert P., Spring J., Schmid V. (1996). The polyp and its medusa: a molecular approach. Sci. Mar. 60, 7–16.
-
- Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K., et al. (2015). Molecular Biology of the Cell, 6th Edn. New York, NY: Garland Science.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

