The structurally disordered paramyxovirus nucleocapsid protein tail domain is a regulator of the mRNA transcription gradient
- PMID: 28168220
- PMCID: PMC5291697
- DOI: 10.1126/sciadv.1602350
The structurally disordered paramyxovirus nucleocapsid protein tail domain is a regulator of the mRNA transcription gradient
Abstract
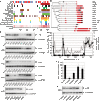
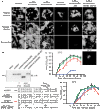
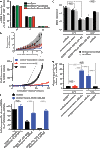
The paramyxovirus RNA-dependent RNA-polymerase (RdRp) complex loads onto the nucleocapsid protein (N)-encapsidated viral N:RNA genome for RNA synthesis. Binding of the RdRp of measles virus (MeV), a paramyxovirus archetype, is mediated through interaction with a molecular recognition element (MoRE) located near the end of the carboxyl-terminal Ntail domain. The structurally disordered central Ntail section is thought to add positional flexibility to MoRE, but the functional importance of this Ntail region for RNA polymerization is unclear. To address this question, we dissected functional elements of Ntail by relocating MoRE into the RNA-encapsidating Ncore domain. Linker-scanning mutagenesis identified a microdomain in Ncore that tolerates insertions. MoRE relocated to Ncore supported efficient interaction with N, MoRE-deficient Ntails had a dominant-negative effect on bioactivity that was alleviated by insertion of MoRE into Ncore, and recombinant MeV encoding N with relocated MoRE grew efficiently and remained capable of mRNA editing. MoRE in Ncore also restored viability of a recombinant lacking the disordered central Ntail section, but this recombinant was temperature-sensitive, with reduced RdRp loading efficiency and a flattened transcription gradient. These results demonstrate that virus replication requires high-affinity RdRp binding sites in N:RNA, but productive RdRp binding is independent of positional flexibility of MoRE and cis-acting elements in Ntail. Rather, the disordered central Ntail section independent of the presence of MoRE in Ntail steepens the paramyxovirus transcription gradient by promoting RdRp loading and preventing the formation of nonproductive polycistronic viral mRNAs. Disordered Ntails may have evolved as a regulatory element to adjust paramyxovirus gene expression.
Keywords: Nucleocapsid; Paramyxovirus; RNA-dependent RNA polymerase; Virus replication; measles virus.
Figures
Similar articles
-
The Unstructured Paramyxovirus Nucleocapsid Protein Tail Domain Modulates Viral Pathogenesis through Regulation of Transcriptase Activity.J Virol. 2018 Mar 28;92(8):e02064-17. doi: 10.1128/JVI.02064-17. Print 2018 Apr 15. J Virol. 2018. PMID: 29437959 Free PMC article.
-
Modulation of Re-initiation of Measles Virus Transcription at Intergenic Regions by PXD to NTAIL Binding Strength.PLoS Pathog. 2016 Dec 9;12(12):e1006058. doi: 10.1371/journal.ppat.1006058. eCollection 2016 Dec. PLoS Pathog. 2016. PMID: 27936158 Free PMC article.
-
The measles virus nucleocapsid protein tail domain is dispensable for viral polymerase recruitment and activity.J Biol Chem. 2013 Oct 11;288(41):29943-53. doi: 10.1074/jbc.M113.503862. Epub 2013 Sep 3. J Biol Chem. 2013. PMID: 24003217 Free PMC article.
-
Structural disorder within paramyxoviral nucleoproteins.FEBS Lett. 2015 Sep 14;589(19 Pt A):2649-59. doi: 10.1016/j.febslet.2015.05.055. Epub 2015 Jun 10. FEBS Lett. 2015. PMID: 26071376 Review.
-
Nucleocapsid structure and function.Curr Top Microbiol Immunol. 2009;329:103-28. doi: 10.1007/978-3-540-70523-9_6. Curr Top Microbiol Immunol. 2009. PMID: 19198564 Review.
Cited by
-
Measles Virus Forms Inclusion Bodies with Properties of Liquid Organelles.J Virol. 2019 Oct 15;93(21):e00948-19. doi: 10.1128/JVI.00948-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31375591 Free PMC article.
-
miRNA-mediated control of exogenous OCT4 during mesenchymal-epithelial transition increases measles vector reprogramming efficiency.Mol Ther Methods Clin Dev. 2021 Nov 29;24:48-61. doi: 10.1016/j.omtm.2021.11.012. eCollection 2022 Mar 10. Mol Ther Methods Clin Dev. 2021. PMID: 34977272 Free PMC article.
-
Structural Description of the Nipah Virus Phosphoprotein and Its Interaction with STAT1.Biophys J. 2020 May 19;118(10):2470-2488. doi: 10.1016/j.bpj.2020.04.010. Epub 2020 Apr 18. Biophys J. 2020. PMID: 32348724 Free PMC article.
-
Recent advancements in the diverse roles of polymerase-associated proteins in the replication and pathogenesis of Newcastle disease virus.Vet Res. 2025 Jan 12;56(1):8. doi: 10.1186/s13567-024-01429-0. Vet Res. 2025. PMID: 39800751 Free PMC article. Review.
-
Nipah Virus Bangladesh Infection Elicits Organ-Specific Innate and Inflammatory Responses in the Marmoset Model.J Infect Dis. 2023 Aug 31;228(5):604-614. doi: 10.1093/infdis/jiad053. J Infect Dis. 2023. PMID: 36869692 Free PMC article.
References
-
- R. A. Lamb, D. Kolakofsky, Paramyxoviridae: The viruses and their replication, in Fields Virology, D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, S. E. Straus, Eds. (Lippincott Williams & Wilkins, ed. 4, 2001), pp. 1305–1340.
-
- G. Enders, in Medical Microbiology, S. Baron, Ed. (The University of Texas Medical Branch at Galveston, 1996). - PubMed
-
- Finch J. T., Gibbs A. J., Observations on the structure of the nucleocapsids of some paramyxoviruses. J. Gen. Virol. 6, 141–150 (1970). - PubMed
-
- Longhi S., Nucleocapsid structure and function. Curr. Top. Microbiol. Immunol. 329, 103–128 (2009). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

