Dynamic assembly of Hda and the sliding clamp in the regulation of replication licensing
- PMID: 28168278
- PMCID: PMC5397184
- DOI: 10.1093/nar/gkx081
Dynamic assembly of Hda and the sliding clamp in the regulation of replication licensing
Abstract
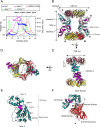
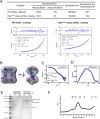
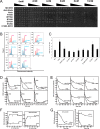
Regulatory inactivation of DnaA (RIDA) is one of the major regulatory mechanisms of prokaryotic replication licensing. In RIDA, the Hda-sliding clamp complex loaded onto DNA directly interacts with adenosine triphosphate (ATP)-bound DnaA and stimulates the hydrolysis of ATP to inactivate DnaA. A prediction is that the activity of Hda is tightly controlled to ensure that replication initiation occurs only once per cell cycle. Here, we determined the crystal structure of the Hda-β clamp complex. This complex contains two pairs of Hda dimers sandwiched between two β clamp rings to form an octamer that is stabilized by three discrete interfaces. Two separate surfaces of Hda make contact with the β clamp, which is essential for Hda function in RIDA. The third interface between Hda monomers occludes the active site arginine finger, blocking its access to DnaA. Taken together, our structural and mutational analyses of the Hda-β clamp complex indicate that the interaction of the β clamp with Hda controls the ability of Hda to interact with DnaA. In the octameric Hda-β clamp complex, the inability of Hda to interact with DnaA is a novel mechanism that may regulate Hda function.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- Kaguni J.M. DnaA: controlling the initiation of bacterial DNA replication and more. Annu. Rev. Microbiol. 2006; 60:351–375. - PubMed
-
- Sutton M.D., Carr K.M., Vicente M., Kaguni J.M.. Escherichia coli DnaA protein. The N-terminal domain and loading of DnaB helicase at the E. coli chromosomal origin. J. Biol. Chem. 1998; 273:34255–34262. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

