The Terminal End Bud: the Little Engine that Could
- PMID: 28168376
- PMCID: PMC5488158
- DOI: 10.1007/s10911-017-9372-0
The Terminal End Bud: the Little Engine that Could
Abstract
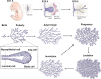
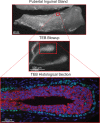
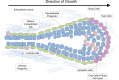
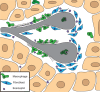
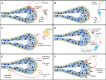
The mammary gland is one of the most regenerative organs in the body, with the majority of development occurring postnatally and in the adult mammal. Formation of the ductal tree is orchestrated by a specialized structure called the terminal end bud (TEB). The TEB is responsible for the production of mature cell types leading to the elongation of the subtending duct. The TEB is also the regulatory control point for basement membrane deposition, branching, angiogenesis, and pattern formation. While the hormonal control of TEB growth is well characterized, the local regulatory factors are less well understood. Recent studies of pubertal outgrowth and ductal elongation have yielded surprising details in regards to ongoing processes in the TEB. Here we summarize the current understanding of TEB biology, discuss areas of future study, and discuss the use of the TEB as a model for the study of breast cancer.
Keywords: Cap cell; Ductal elongation; Mammary gland; Terminal end bud.
Conflict of interest statement
This work was supported by National Science Foundation grant DMS-126375. Michael T. Lewis is a Limited Partner in StemMed Ltd. and a Manager in StemMed Holdings L.L.C.
Figures
References
-
- Sakakura T, et al. Biology of mammary fat pad in fetal mouse: capacity to support development of various fetal epithelia in vivo. Development. 1987;100(3):421–430. - PubMed
-
- Dunbar ME, et al. Parathyroid hormone-related protein signaling is necessary for sexual dimorphism during embryonic mammary development. Development. 1999;126(16):3485–3493. - PubMed
-
- Hogg NA, Harrison CJ, Tickle C. Lumen formation in the developing mouse mammary gland. J Embryol Exp Morphol. 1983;73:39–57. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

