Vaccination of calves with Mycobacterium bovis Bacillus Calmette-Guerin reduces the frequency and severity of lesions of bovine tuberculosis under a natural transmission setting in Ethiopia
- PMID: 28168855
- PMCID: PMC5811905
- DOI: 10.1111/tbed.12618
Vaccination of calves with Mycobacterium bovis Bacillus Calmette-Guerin reduces the frequency and severity of lesions of bovine tuberculosis under a natural transmission setting in Ethiopia
Abstract
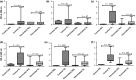
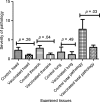
Bovine tuberculosis (bTB) is highly prevalent in intensive dairy farms of the urban "milk-sheds" in Ethiopia, and vaccination could be a cost-effective disease control strategy. In the present study, the efficacy of Bacillus Calmette-Guerin (BCG) to protect against bTB was assessed in Holstein-Friesian calves in a natural transmission setting. Twenty-three 2-week-old calves were subcutaneously vaccinated with BCG Danish SSI strain 1331, and matched 26 calves were injected with placebo. Six weeks later, calves were introduced into a herd of M. bovis-infected animals (reactors) and kept in contact with them for 1 year. In vitro and in vivo immunological tests were performed to assess immune responses post-vaccination and during exposure. Successful vaccine uptake was confirmed by tuberculin skin test and IFN-γ responses in vaccinated calves. The kinetics of IFN-γ responses to early secretory antigen target 6 and culture filtrate protein 10 (ESAT6 and CFP10, respectively) and tuberculin skin test responses post-exposure suggested that the animals were infected early after being placed in contact with the infected herd as immunological signs of infection were measurable between 2 and 4 months post-initial exposure. Protection was determined by comparing gross and microscopic pathology and bacteriological burden between vaccinated and control calves. BCG vaccination reduced the proportions of tissues with visible pathology in vaccinates compared to control calves by 49% (p < .001) with 56%, 43%, 72%, and 38% reductions in the proportion of lesioned tisues in head, thoracic, abdominal lymph nodes, and lungs, respectively (p-values .029-.0001). In addition, the lesions were less severe grossly and microscopically in vaccinated calves than in non-vaccinated calves (p < .05). The reduction in the overall incidence rates of bTB was 23%, 28%, and 33% on the basis of the absence of gross pathology, M. bovis culture positivity, and histopathology, respectively, in vaccinated animals. In conclusion, BCG vaccination reduced the frequency and severity of the pathology of bTB significantly, which is likely to reduce onwards transmission of the disease.
Keywords: BCG vaccination; bovine tuberculosis; natural transmission.
© 2017 The Authors. Transboundary and Emerging Diseases Published by Blackwell Verlag GmbH.
Figures



References
-
- Ameni, G. , Aseffa, A. , Engers, H. , Young, D. B. , Gordon, S. V. , Hewinson, G. R. , & Vordermeier, M. H. (2007). High prevalence and increased severity of pathology of bovine tuberculosis in Holsteins as compared to zebu breeds in central highlands of Ethiopia. Clinical and Vaccine Immunology, 14(10), 1356–1361. - PMC - PubMed
-
- Aranday‐Cortes, E. , Bull, N. C. , Villarreal‐Ramos, B. , Gough, J. , Hicks, D. , Ortiz‐Peláez, A. , … Salguero, F. J. (2013). Upregulation of IL‐17A, CXCL9 and CXCL10 in early‐stage granulomas induced by Mycobacterium bovis in cattle. Transboundary and Emerging Diseases, 60, 525–537. - PubMed
-
- CSA . (2011). Report on Livestock and Livestock Characteristics. Agricultural Sample Survey 2011/2012. Central Statistics Agency of Ethiopia, Addis Ababa, Ethiopia
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

