Molecular determinant of the effects of hydrostatic pressure on protein folding stability
- PMID: 28169271
- PMCID: PMC5309723
- DOI: 10.1038/ncomms14561
Molecular determinant of the effects of hydrostatic pressure on protein folding stability
Abstract
Hydrostatic pressure is an important environmental variable that plays an essential role in biological adaptation for many extremophilic organisms (for example, piezophiles). Increase in hydrostatic pressure, much like increase in temperature, perturbs the thermodynamic equilibrium between native and unfolded states of proteins. Experimentally, it has been observed that increase in hydrostatic pressure can both increase and decrease protein stability. These observations suggest that volume changes upon protein unfolding can be both positive and negative. The molecular details of this difference in sign of volume changes have been puzzling the field for the past 50 years. Here we present a comprehensive thermodynamic model that provides in-depth analysis of the contribution of various molecular determinants to the volume changes upon protein unfolding. Comparison with experimental data shows that the model allows quantitative predictions of volume changes upon protein unfolding, thus paving the way to proteome-wide computational comparison of proteins from different extremophilic organisms.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

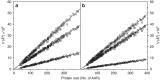
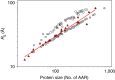

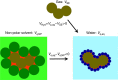


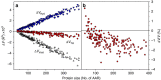

References
-
- Horikoshi K. Barophiles: deep-sea microorganisms adapted to an extreme environment. Curr. Opin. Microbiol. 1, 291–295 (1998). - PubMed
-
- Lauro F. M. & Bartlett D. H. Prokaryotic lifestyles in deep sea habitats. Extremophiles 12, 15–25 (2008). - PubMed
-
- Yayanos A. A. Microbiology to 10,500 meters in the deep sea. Annu. Rev. Microbiol. 49, 777–805 (1995). - PubMed
-
- Robb F. T. & Clark D. S. Adaptation of proteins from hyperthermophiles to high pressure and high temperature. J. Mol. Microbiol. Biotechnol. 1, 101–105 (1999). - PubMed
-
- Seki K. & Toyoshima M. Preserving tardigrades under pressure. Nature 395, 853–854 (1998).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

