A Randomized Phase III Clinical Trial of Plecanatide, a Uroguanylin Analog, in Patients With Chronic Idiopathic Constipation
- PMID: 28169285
- PMCID: PMC5415706
- DOI: 10.1038/ajg.2016.611
A Randomized Phase III Clinical Trial of Plecanatide, a Uroguanylin Analog, in Patients With Chronic Idiopathic Constipation
Abstract
Objectives: This study assessed the efficacy and safety of plecanatide, a guanylate cyclase-C (GC-C) agonist and the first uroguanylin analog approved for the treatment of chronic idiopathic constipation (CIC).
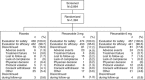
Methods: This phase III, multicenter, double-blind, placebo-controlled study randomized 1,394 patients with CIC. Patients received either plecanatide (3 or 6 mg) or placebo, orally, once daily, for 12 weeks. The primary efficacy endpoint was the percentage of patients who were durable overall complete spontaneous bowel movement (CSBM) responders over the 12-week treatment period. Patients were instructed to record their daily bowel movements, stool consistency scores, and abdominal symptoms in an electronic diary. Treatment-emergent adverse events (AEs) were collected.
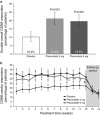
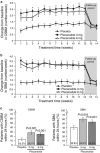
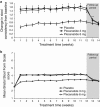
Results: Each dose of plecanatide resulted in a significantly greater percentage of durable overall CSBM responders (21.0%, 3 mg; 19.5%, 6 mg) as compared with placebo (10.2%; P<0.001 for both). Plecanatide (3 and 6 mg) also significantly increased mean weekly CSBM frequency from baseline (increase of 2.5 and 2.2/week, respectively) vs. placebo (1.2/week; P<0.001 for both) and mean weekly spontaneous bowel movement frequency (increase of 3.2 and 3.1/week, respectively) vs. placebo (1.3/week; P<0.001, for both) over the 12-week treatment period. Both plecanatide doses significantly improved all secondary and additional efficacy endpoints. The most common AE, diarrhea, occurred in 1.3% (placebo), 5.9% (3 mg) and 5.7% (6 mg) of patients.
Conclusions: Plecanatide significantly improved constipation and its related symptoms with a low rate of adverse events. These results suggest that plecanatide will be a useful treatment option in the management of CIC. ClinicalTrials.gov: NCT01982240.
Conflict of interest statement
Figures
Comment in
-
Response to Miner et al.Am J Gastroenterol. 2017 Nov;112(11):1750-1751. doi: 10.1038/ajg.2017.362. Am J Gastroenterol. 2017. PMID: 29109497 Free PMC article. No abstract available.
-
Response to Currie et al.Am J Gastroenterol. 2017 Nov;112(11):1751. doi: 10.1038/ajg.2017.384. Am J Gastroenterol. 2017. PMID: 29109504 No abstract available.
References
-
- Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol 2011;106:1582–1591. - PubMed
-
- Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004;99:750–759. - PubMed
-
- American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol 2005;100 (Suppl 1): S1–S4. - PubMed
-
- Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther 2007;25:599–608. - PubMed
-
- Bharucha AE, Dorn SD, Lembo A et al. American Gastroenterological Association medical position statement on constipation. Gastroenterology 2013;144:211–217. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

