Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress
- PMID: 28169318
- PMCID: PMC5294586
- DOI: 10.1038/srep42039
Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress
Abstract
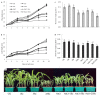
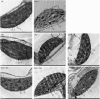
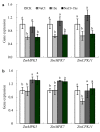
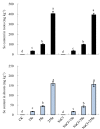
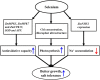
The mechanism of selenium-mediated salt tolerance has not been fully clarified. This study investigated the possible role of selenium (Se) in regulating maize salt tolerance. A pot experiment was conducted to investigate the role of Se (0, 1, 5 and 25 μM Na2SeO3) in photosynthesis, antioxidative capacity and ion homeostasis in maize under salinity. The results showed that Se (1 μM) relieved the salt-induced inhibitory effects on the plant growth and development of 15-day-old maize plants. Se application (1 μM) also increased the net photosynthetic rate and alleviated the damage to chloroplast ultrastructure induced by NaCl. The superoxide dismutase (SOD) and ascorbate peroxidase (APX) activities were increased, and ZmMPK5, ZmMPK7 and ZmCPK11 were markedly up-regulated in the roots of Se-treated plants, likely contributing to the improvement of antioxidant defence systems under salinity. Moreover, 1 μM Se increased K+ in the shoots while decreasing Na+ in the roots, indicating that Se up-regulates ZmNHX1 in the roots, which may be involved in Na+ compartmentalisation under salinity. The findings from this single experiment require repetition together with measurement of reactive oxygen species (ROS), but nevertheless suggest that exogenous Se alleviates salt stress in maize via the improvement of photosynthetic capacity, the activities of antioxidant enzymes and the regulation of Na+ homeostasis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Exogenous 2-(3,4-Dichlorophenoxy) triethylamine alleviates salinity stress in maize by enhancing photosynthetic capacity, improving water status and maintaining K+/Na+ homeostasis.BMC Plant Biol. 2020 Jul 23;20(1):348. doi: 10.1186/s12870-020-02550-w. BMC Plant Biol. 2020. PMID: 32703161 Free PMC article.
-
Exogenous Melatonin Alleviates NaCl Injury by Influencing Stomatal Morphology, Photosynthetic Performance, and Antioxidant Balance in Maize.Int J Mol Sci. 2024 Sep 19;25(18):10077. doi: 10.3390/ijms251810077. Int J Mol Sci. 2024. PMID: 39337563 Free PMC article.
-
ZmCPK11 is involved in abscisic acid-induced antioxidant defence and functions upstream of ZmMPK5 in abscisic acid signalling in maize.J Exp Bot. 2013 Feb;64(4):871-84. doi: 10.1093/jxb/ers366. Epub 2012 Dec 26. J Exp Bot. 2013. PMID: 23268839 Free PMC article.
-
Effects of Salinity Stress on Chloroplast Structure and Function.Cells. 2021 Aug 7;10(8):2023. doi: 10.3390/cells10082023. Cells. 2021. PMID: 34440792 Free PMC article. Review.
-
Photosynthetic machinery under salinity stress: Trepidations and adaptive mechanisms.Photosynthetica. 2023 Mar 14;61(1):73-93. doi: 10.32615/ps.2023.002. eCollection 2023. Photosynthetica. 2023. PMID: 39650121 Free PMC article. Review.
Cited by
-
Plant Nutrition: An Effective Way to Alleviate Abiotic Stress in Agricultural Crops.Int J Mol Sci. 2022 Jul 31;23(15):8519. doi: 10.3390/ijms23158519. Int J Mol Sci. 2022. PMID: 35955651 Free PMC article. Review.
-
Selenium uptake, translocation, subcellular distribution and speciation in winter wheat in response to phosphorus application combined with three types of selenium fertilizer.BMC Plant Biol. 2023 Apr 27;23(1):224. doi: 10.1186/s12870-023-04227-6. BMC Plant Biol. 2023. PMID: 37101116 Free PMC article.
-
Enhancing salt stress tolerance in Carthamus tinctorius L. through selenium soil treatment: anatomical, biochemical, and physiological insights.BMC Plant Biol. 2025 Jan 24;25(1):100. doi: 10.1186/s12870-025-06078-9. BMC Plant Biol. 2025. PMID: 39856597 Free PMC article.
-
Melatonin Participates in Selenium-Enhanced Cold Tolerance of Cucumber Seedlings.Front Plant Sci. 2021 Dec 22;12:786043. doi: 10.3389/fpls.2021.786043. eCollection 2021. Front Plant Sci. 2021. PMID: 35003171 Free PMC article.
-
Effect of Rice Grain (Oryza sativa L.) Enrichment with Selenium on Foliar Leaf Gas Exchanges and Accumulation of Nutrients.Plants (Basel). 2021 Feb 3;10(2):288. doi: 10.3390/plants10020288. Plants (Basel). 2021. PMID: 33546440 Free PMC article.
References
-
- Zhu J. K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 6, 441–445 (2003). - PubMed
-
- Munns R. & Tester M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 59, 651–681 (2008). - PubMed
-
- Jiang C. et al.. Overexpression of Arabidopsis thaliana Na+/H+ antiporter gene enhanced salt resistance in transgenic poplar (Populus × euramericana ‘Neva’). Trees 26, 685–694 (2012).
-
- Jiang C. et al.. Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta. Physiol. Plant. 38, doi: 10.1007/s11738-016-2101-2 (2016). - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

