Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation
- PMID: 28169833
- PMCID: PMC5370189
- DOI: 10.7554/eLife.18834
Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation
Erratum in
-
Correction: Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation.Elife. 2018 Feb 15;7:e35178. doi: 10.7554/eLife.35178. Elife. 2018. PMID: 29446753 Free PMC article. No abstract available.
Abstract
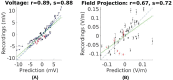
Transcranial electric stimulation aims to stimulate the brain by applying weak electrical currents at the scalp. However, the magnitude and spatial distribution of electric fields in the human brain are unknown. We measured electric potentials intracranially in ten epilepsy patients and estimated electric fields across the entire brain by leveraging calibrated current-flow models. When stimulating at 2 mA, cortical electric fields reach 0.8 V/m, the lower limit of effectiveness in animal studies. When individual whole-head anatomy is considered, the predicted electric field magnitudes correlate with the recorded values in cortical (r = 0.86) and depth (r = 0.88) electrodes. Accurate models require adjustment of tissue conductivity values reported in the literature, but accuracy is not improved when incorporating white matter anisotropy or different skull compartments. This is the first study to validate and calibrate current-flow models with in vivo intracranial recordings in humans, providing a solid foundation to target stimulation and interpret clinical trials.
Keywords: computational current-flow model; human; intracranial recordings; neuroscience; transcranial electric stimulation.
Conflict of interest statement
MB: Has significant interest in Soterix Medical Inc. which commercializes hardware and software for TES. He is listed as inventors on patents (U.S. Patent application No.13/264,142) related to TES.
LCP: Has significant interest in Soterix Medical Inc. which commercializes hardware and software for TES. He is listed as inventors on patents (U.S. Patent application No.13/264,142) related to TES.
The other authors declare that no competing interests exist.
Figures








Comment in
-
Transcranial electric stimulation seen from within the brain.Elife. 2017 Mar 28;6:e25812. doi: 10.7554/eLife.25812. Elife. 2017. PMID: 28350293 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

