Erythrocytes retain hypoxic adenosine response for faster acclimatization upon re-ascent
- PMID: 28169986
- PMCID: PMC5309698
- DOI: 10.1038/ncomms14108
Erythrocytes retain hypoxic adenosine response for faster acclimatization upon re-ascent
Abstract
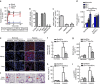
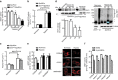
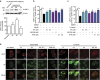
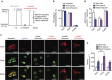
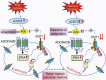
Faster acclimatization to high altitude upon re-ascent is seen in humans; however, the molecular basis for this enhanced adaptive response is unknown. We report that in healthy lowlanders, plasma adenosine levels are rapidly induced by initial ascent to high altitude and achieved even higher levels upon re-ascent, a feature that is positively associated with quicker acclimatization. Erythrocyte equilibrative nucleoside transporter 1 (eENT1) levels are reduced in humans at high altitude and in mice under hypoxia. eENT1 deletion allows rapid accumulation of plasma adenosine to counteract hypoxic tissue damage in mice. Adenosine signalling via erythrocyte ADORA2B induces PKA phosphorylation, ubiquitination and proteasomal degradation of eENT1. Reduced eENT1 resulting from initial hypoxia is maintained upon re-ascent in humans or re-exposure to hypoxia in mice and accounts for erythrocyte hypoxic memory and faster acclimatization. Our findings suggest that targeting identified purinergic-signalling network would enhance the hypoxia adenosine response to counteract hypoxia-induced maladaptation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Global expression profiling and pathway analysis in two different population groups in relation to high altitude.Funct Integr Genomics. 2019 Jan;19(1):205-215. doi: 10.1007/s10142-018-0637-5. Epub 2018 Oct 19. Funct Integr Genomics. 2019. PMID: 30341547
-
Beneficial Role of Erythrocyte Adenosine A2B Receptor-Mediated AMP-Activated Protein Kinase Activation in High-Altitude Hypoxia.Circulation. 2016 Aug 2;134(5):405-21. doi: 10.1161/CIRCULATIONAHA.116.021311. Circulation. 2016. PMID: 27482003 Free PMC article.
-
Erythrocyte adaptive metabolic reprogramming under physiological and pathological hypoxia.Curr Opin Hematol. 2020 May;27(3):155-162. doi: 10.1097/MOH.0000000000000574. Curr Opin Hematol. 2020. PMID: 32141895 Free PMC article. Review.
-
Elevated ecto-5'-nucleotidase-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension.Circ Res. 2013 May 24;112(11):1466-78. doi: 10.1161/CIRCRESAHA.111.300166. Epub 2013 Apr 12. Circ Res. 2013. PMID: 23584256 Free PMC article.
-
Erythrocyte purinergic signaling components underlie hypoxia adaptation.J Appl Physiol (1985). 2017 Oct 1;123(4):951-956. doi: 10.1152/japplphysiol.00155.2017. Epub 2017 Jun 1. J Appl Physiol (1985). 2017. PMID: 28572494 Free PMC article. Review.
Cited by
-
Exercise vs. high altitude therapy.J Sport Health Sci. 2018 Jan;7(1):125. doi: 10.1016/j.jshs.2017.07.002. Epub 2017 Jul 4. J Sport Health Sci. 2018. PMID: 30356491 Free PMC article. No abstract available.
-
Adenosine at the Interphase of Hypoxia and Inflammation in Lung Injury.Front Immunol. 2021 Jan 14;11:604944. doi: 10.3389/fimmu.2020.604944. eCollection 2020. Front Immunol. 2021. PMID: 33519814 Free PMC article. Review.
-
Acute Cycling Exercise Induces Changes in Red Blood Cell Deformability and Membrane Lipid Remodeling.Int J Mol Sci. 2021 Jan 18;22(2):896. doi: 10.3390/ijms22020896. Int J Mol Sci. 2021. PMID: 33477427 Free PMC article.
-
Circulating MicroRNA Markers for Pulmonary Hypertension in Supervised Exercise Intervention and Nightly Oxygen Intervention.Front Physiol. 2018 Jul 25;9:955. doi: 10.3389/fphys.2018.00955. eCollection 2018. Front Physiol. 2018. PMID: 30090067 Free PMC article.
-
Global expression profiling and pathway analysis in two different population groups in relation to high altitude.Funct Integr Genomics. 2019 Jan;19(1):205-215. doi: 10.1007/s10142-018-0637-5. Epub 2018 Oct 19. Funct Integr Genomics. 2019. PMID: 30341547
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

