Theta and beta synchrony coordinate frontal eye fields and anterior cingulate cortex during sensorimotor mapping
- PMID: 28169987
- PMCID: PMC5309702
- DOI: 10.1038/ncomms13967
Theta and beta synchrony coordinate frontal eye fields and anterior cingulate cortex during sensorimotor mapping
Abstract
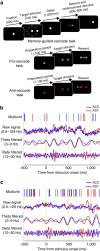
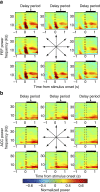
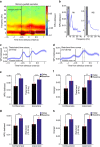
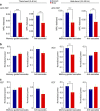
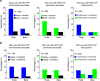
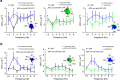
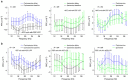
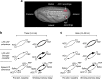
The frontal eye fields (FEFs) and the anterior cingulate cortex (ACC) are commonly coactivated for cognitive saccade tasks, but whether this joined activation indexes coordinated activity underlying successful guidance of sensorimotor mapping is unknown. Here we test whether ACC and FEF circuits coordinate through phase synchronization of local field potential and neural spiking activity in macaque monkeys performing memory-guided and pro- and anti-saccades. We find that FEF and ACC showed prominent synchronization at a 3-9 Hz theta and a 12-30 Hz beta frequency band during the delay and preparation periods with a strong Granger-causal influence from ACC to FEF. The strength of theta- and beta-band coherence between ACC and FEF but not variations in power predict correct task performance. Taken together, the results support a role of ACC in cognitive control of frontoparietal networks and suggest that narrow-band theta and to some extent beta rhythmic activity indexes the coordination of relevant information during periods of enhanced control demands.
Conflict of interest statement
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors declare no competing financial interests.
Figures
References
-
- Wurtz R. H & Mohler C. W. Enhancement of visual responses in monkey striate cortex and frontal eye fields. J. Neurophysiol. 39, 766–772 (1976). - PubMed
-
- Bruce C. J., Goldberg M. E., Bushnell M. C. & Stanton G. B. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J. Neurophysiol. 54, 714–734 (1985). - PubMed
-
- Moore T. & Armstrong K. M. Selective gating of visual signals by microstimulation of frontal cortex. Nature 421, 370–373 (2003). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

