Topical treatments for blepharokeratoconjunctivitis in children
- PMID: 28170093
- PMCID: PMC6464561
- DOI: 10.1002/14651858.CD011965.pub2
Topical treatments for blepharokeratoconjunctivitis in children
Abstract
Background: Blepharokeratoconjunctivitis (BKC) is a type of inflammation of the surface of the eye and eyelids that involves changes of the eyelids, dysfunction of the meibomian glands, and inflammation of the conjunctiva and cornea. Chronic inflammation of the cornea can lead to scarring, vascularisation and opacity. BKC in children can cause significant symptoms including irritation, watering, photophobia and loss of vision from corneal opacity, refractive error or amblyopia.Treatment of BKC is directed towards modification of meibomian gland disease and the bacterial flora of lid margin and conjunctiva, and control of ocular surface inflammation. Although both topical and systemic treatments are used to treat people with BKC, this Cochrane review focuses on topical treatments.
Objectives: To assess and compare data on the efficacy and safety of topical treatments (including antibiotics, steroids, immunosuppressants and lubricants), alone or in combination, for BKC in children from birth to 16 years.
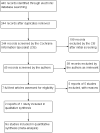
Search methods: We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 6), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE ( January 1946 to 11 July 2016), Embase (January 1980 to 11 July 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 11 July 2016. We searched the reference lists of identified reports and the Science Citation Index to identify any additional reports of studies that met the inclusion criteria.
Selection criteria: We searched for randomised controlled trials that involved topical treatments in children up to 16 years of age with a clinical diagnosis of BKC. We planned to include studies that evaluated a single topical medication versus placebo, a combination of treatments versus placebo, and those that compared two or multiple active treatments. We planned to include studies in which participants received additional treatments, such as oral antibiotics, oral anti-inflammatories, warm lid compresses and lid margin cleaning.
Data collection and analysis: Two review authors independently screened the results of the literature search (titles and abstracts) to identify studies that met the inclusion criteria of the review and applied standards as expected for Cochrane reviews. We graded the certainty of the evidence using GRADE.
Main results: We included one study from the USA that met the inclusion criteria. In the study, 137 children aged zero to six years old with blepharoconjunctivitis were randomised to treatment in one of four trial arms (loteprednol etabonate/tobramycin combination, loteprednol etabonate alone, tobramycin alone or placebo) for 15 days, with assessments on days 1, 3, 7 and 15. We judged the study to be at high risk of attrition bias and bias due to selective outcome reporting. The study did not report the number of children with improvement in symptoms nor with total or partial success as measured by changes in clinical symptoms.All children showed a reduction in blepharoconjunctivitis grade score, but there was no evidence of important differences between groups. Visual acuity was not fully reported but the authors stated that there was no change in visual acuity in any of the treatment groups. The study reported ocular and non ocular adverse events but was underpowered to detect differences between the groups. Ocular adverse events were as follows: loteprednol/tobramycin 1/34 (eye pain); loteprednol 4/35 (eye pain, conjunctivitis, eye discharge, eye inflammation); tobramycin 0/34; placebo (vehicle) 0/34. The evidence was limited for all these outcomes and we judged it to be very low certainty.There was no information on clinical signs (aside from grade score), disease progression or quality of life.
Authors' conclusions: There is no high-quality evidence of the safety and efficacy of topical treatments for BKC, which resulted in uncertainty about the indications and effectiveness of topical treatment. Clinical trials are required to test efficacy and safety of current and any future treatments. Outcome measures need to be developed which can capture both objective clinical and patient-reported aspects of the condition and treatments.
Conflict of interest statement
MO'G has no known conflicts of interest. CB has no known conflicts of interest. MH has no known conflicts of interest. FL has no known conflicts of interest. ST has no known conflicts of interest. ADN has no known conflicts of interest.
Figures
Update of
- doi: 10.1002/14651858.CD011965
References
References to studies included in this review
Comstock 2012 {published data only (unpublished sought but not used)}
-
- Comstock TL, Paterno MR, Bateman KM, DeCory HH, Gearinger M. Safety and tolerability of loteprednol etabonate 0.5% and tobramycin 0.3% ophthalmic suspension in pediatric subjects. Paediatric Drugs 2012;14(2):119‐30. - PubMed
References to studies excluded from this review
Bloom 1994 {published data only}
-
- Bloom PA, Leeming JP, Power W, Laidlaw DA, Collum LM, Easty DL. Topical ciprofloxacin in the treatment of blepharitis and blepharoconjunctivitis. European Journal of Ophthalmology 1994;4(1):6‐12. - PubMed
Chen 2012 {published data only}
-
- Chen M, Gong L, Sun X, Gu Y, He X, Qu J, et al. A multicenter, randomized, parallel‐group, clinical trial comparing the safety and efficacy of loteprednol etabonate 0.5%/tobramycin 0.3% with dexamethasone 0.1%/tobramycin 0.3% in the treatment of Chinese patients with blepharokeratoconjunctivitis. Current Medical Research and Opinion 2012;28(3):385‐94. - PubMed
Hosseini 2013 {published data only}
Shulman 1982 {published data only}
-
- Shulman J, Koreman N, Hirshman M, Samson C, Trochelman L. A double‐blind, comparative clinical trial of a new steroid, anti‐infective ophthalmic ointment for chronic staphylococcal blepharoconjunctivitis. Ocular Therapy and Surgery 1982;1:192‐7.
White 2008 {published data only}
-
- White EM, Macy JI, Bateman KM, Comstock TL. Comparison of the safety and efficacy of loteprednol 0.5%/tobramycin 0.3% with dexamethasone 0.1%/tobramycin 0.3% in the treatment of blepharokeratoconjunctivitis. Current Medical Research and Opinion 2008;24(1):287‐96. - PubMed
Additional references
Afandi 2003
-
- Afandi B, Toumeh MS, Saadi HF. Cushing's syndrome caused by unsupervised use of ocular glucocorticoids. Endocrine Practice 2003;9(6):526‐9. - PubMed
Asbell 2011
Auw‐Hädrich 2009
-
- Auw‐Hädrich C, Reinhard T. Treatment of chronic blepharokeratoconjunctivitis with local calcineurin inhibitors. Ophthalmologe 2009;106(7):635‐8. - PubMed
Bondalapati 2014
-
- Bondalapati S, Cabrera MT. Sub‐tenon triamcinolone acetonide injections for topical medication intolerance in chronic blepharokeratoconjunctivitis. Cornea 2014;33(9):999‐1001. - PubMed
Bron 2003
-
- Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003;22(7):640‐50. - PubMed
Cehajic‐Kapetanovic 2010
-
- Cehajic‐Kapetanovic J, Kwartz J. Augmentin duo™ in the treatment of childhood blepharokeratoconjunctivitis. Journal of Pediatric Ophthalmology and Strabismus 2010;47(6):356‐60. - PubMed
Chang 2012
Chiang 2006
-
- Chiang MY, Sarkar M, Koppens JM, Milles J, Shah P. Exogenous Cushing's syndrome and topical ocular steroids. Eye 2006;20(6):725‐7. - PubMed
Choi 2013
Comstock 2010
-
- Comstock TL, Holland EJ. Loteprednol and tobramycin in combination: a review of their impact on current treatment regimens. Expert Opinion on Pharmacotherapy 2010;11(5):843‐52. - PubMed
Comstock 2012b
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman DG editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Doan 2007
-
- Doan S, Gabison EE, Nghiem‐Buffet S, Abitbol O, Gatinel D, Hoang‐Xuan T. Long‐term visual outcome of childhood blepharokeratoconjunctivitis. American Journal of Ophthalmology 2007;143(3):528‐9. - PubMed
Doan 2013
Dougherty 2011
Elbaz 2015
-
- Elbaz U, Mireskandari K, Shen C, Ali A. Corneal fine needle diathermy with adjuvant bevacizumab to treat corneal neovascularization in children. Cornea 2015;34(7):773‐7. - PubMed
Elder 1997
-
- Elder MJ, Bernauer W. Monitoring of activity and progression in cicatrising conjunctivitis. Developments in Ophthalmology 1997;28:111‐22. - PubMed
Everett 1995
-
- Everett SL, Kowalski RP, Karenchak LM, Landsittel D, Day R, Gordon YJ. An in vitro comparison of the susceptibilities of bacterial isolates from patients with conjunctivitis and blepharitis to newer and established topical antibiotics. Cornea 1995;14(4):382‐7. - PubMed
Farpour 2001
-
- Farpour B, McClellan KA. Diagnosis and management of chronic blepharokeratoconjunctivitis in children. Journal of Pediatric Ophthalmology and Strabismus 2001;38(4):207‐12. - PubMed
Foulks 2003
-
- Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocular Surface 2003;1(3):107‐26. - PubMed
Geerling 2011
-
- Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O'Brien T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Investigative Ophthalmology and Visual Science 2011;52(4):2050‐64. - PMC - PubMed
GRADEpro 2014 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 14 June 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Gupta 2010
-
- Gupta N, Dhawan A, Beri S, D'Souza P. Clinical spectrum of pediatric blepharokeratoconjunctivitis. Journal of AAPOS 2010;14(6):527‐9. - PubMed
Hamada 2012
-
- Hamada S, Khan I, Denniston AK, Rauz S. Childhood blepharokeratoconjunctivitis: characterising a severe phenotype in white adolescents. British Journal of Ophthalmology 2012;96(7):949‐55. - PubMed
Hamada 2013
-
- Hamada S, Nischal K, Evans J. The activity and damage of blepharokeratoconjunctivitis in children. Journal of AAPOS 2013;17(1):e16.
Hammersmith 2005
-
- Hammersmith KM, Cohen EJ, Blake TD, Laibson PR, Rapuano CJ. Blepharokeratoconjunctivitis in children. Archives of Ophthalmology 2005;123(12):1667‐70. - PubMed
Hammersmith 2015
-
- Hammersmith KM. Blepharokeratoconjunctivitis in children. Current Opinion in Ophthalmology 2015;26(4):301‐5. - PubMed
Higgins 2011a
-
- Higgins JP, Deeks JJ editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Altman DG editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ismail 2012
Jones 2007
-
- Jones SM, Weinstein JM, Cumberland P, Klein N, Nischal KK. Visual outcome and corneal changes in children with chronic blepharokeratoconjunctivitis. Ophthalmology 2007;114(12):2271‐80. - PubMed
Joseph 2005
-
- Joseph MA, Kaufman HE, Insler M. Topical tacrolimus ointment for treatment of refractory anterior segment inflammatory disorders. Cornea 2005;24(4):417‐20. - PubMed
Kacmaz 2010
Klein 1997
-
- Klein JO. History of macrolide use in pediatrics. Pediatric Infectious Disease Journal 1997;16(4):427‐31. - PubMed
Krupin 1976
-
- Krupin T, Mandell AI, Podos SM, Becker B. Topical corticosteroid therapy and pituitary‐adrenal function. Archives of Ophthalmology 1976;94(6):919‐20. - PubMed
Kunert 2000
-
- Kunert KS, Tisdale AS, Stern ME, Smith JA, Gipson IK. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Archives of Ophthalmology 2000;118(11):1489‐96. - PubMed
Li 2006
Li 2010
Lindsley 2012
Luo 2004
-
- Luo L, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP‐9 and activates MAPK signaling pathways on the ocular surface. Investigative Ophthalmology and Visual Science 2004;45(12):4293‐301. - PubMed
Mehta 2006
-
- Mehta JS, Sagoo MS, Tuft SJ. Subconjunctival crystals in paediatric blepharokeratoconjunctivitis. Acta Ophthalmologica Scandinavica 2006;84(4):557‐8. - PubMed
Meisler 2000
-
- Meisler DM, Raizman MB, Traboulsi EI. Oral erythromycin treatment for childhood blepharokeratitis. Journal of AAPOS 2000;4(6):379‐80. - PubMed
Murphy 2008
-
- Murphy BS, Sundareshan V, Cory TJ, Hayes D, Anstead MI, Feola DJ. Azithromycin alters macrophage phenotype. Journal of Antimicrobial Chemotherapy 2008;61(3):554‐60. - PubMed
Nelson 2011
Nichols 2011
O'Gallagher 2016
Perry 2006
-
- Perry HD, Doshi‐Carnevale S, Donnenfeld ED, Solomon R, Biser SA, Bloom AH. Efficacy of commercially available topical cyclosporine A 0.05% in the treatment of meibomian gland dysfunction. Cornea 2006;25(2):171‐5. - PubMed
Prabhasawat 2012
-
- Prabhasawat P, Tesavibul N, Mahawong W. A randomized double‐masked study of 0.05% cyclosporine ophthalmic emulsion in the treatment of meibomian gland dysfunction. Cornea 2012;31(12):1386‐93. - PubMed
Qiao 2013
Raskin 1992
-
- Raskin EM, Speaker MG, Laibson PR. Blepharitis. Infectious Disease Clinics of North America 1992;6(4):777‐87. - PubMed
Ratcliffe 2011
-
- Ratcliffe J, Couzner L, Flynn T, Sawyer M, Stevens K, Brazier J, et al. Valuing Child Health Utility 9D health states with a young adolescent sample: a feasibility study to compare best‐worst scaling discrete‐choice experiment, standard gamble and time trade‐off methods. Applied Health Economics and Health Policy 2011;9(1):15‐27. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robert 2001
-
- Robert PY, Adenis JP. Comparative review of topical ophthalmic antibacterial preparations. Drugs 2001;61(2):175‐85. - PubMed
Rodríguez‐Garcia 2016
Rubin 2006
-
- Rubin M, Rao SN. Efficacy of topical cyclosporin 0.05% in the treatment of posterior blepharitis. Journal of Ocular Pharmacology and Therapeutics 2006;22(1):47‐53. - PubMed
Sacchetti 2007
-
- Sacchetti M, Baiardini I, Lambiase A, Aronni S, Fassio O, Gramiccioni C, et al. Development and testing of the quality of life in children with vernal keratoconjunctivitis questionnaire. American Journal of Ophthalmology 2007;144(4):557‐63. - PubMed
Sadrai 2011
Schechter 2009
-
- Schechter BA, Katz RS, Friedman LS. Efficacy of topical cyclosporine for the treatment of ocular rosacea. Advances in Therapy 2009;26(6):651‐9. - PubMed
Singer 1988
Sotozono 2007
-
- Sotozono C, Ang LP, Koizumi N, Higashihara H, Ueta M, Inatomi T, et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens‐Johnson syndrome. Ophthalmology 2007;114(7):1294‐302. - PubMed
Steelman 2001
-
- Steelman J, Kappy M. Adrenal suppression and growth retardation from ocular corticosteroids. Journal of Pediatric Ophthalmology and Strabismus 2001;38(3):177‐8. - PubMed
Stevenson 2000
-
- Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate‐to‐severe dry eye disease: a dose‐ranging, randomized trial. Ophthalmology 2000;107(5):967‐74. - PubMed
Suzuki 2011
-
- Suzuki M, Massingale ML, Ye F, Godbold J, Elfassy T, Vallabhajosyula M, et al. Tear osmolarity as a biomarker for dry eye disease severity. Investigative Ophthalmology and Visual Science 2011;51(9):4557‐61. - PubMed
Suzuki 2015
-
- Suzuki T, Teramukai S, Kinoshita S. Meibomian glands and ocular surface inflammation. The Ocular Surface 2015;13(2):133‐49. - PubMed
Tatlipinar 2005
Teo 2012
-
- Teo L, Mehta JS, Htoon HM, Tan DT. Severity of pediatric blepharokeratoconjunctivitis in Asian eyes. American Journal of Ophthalmology 2012;153(3):564‐70.e.1. - PubMed
Tomlinson 2011
Torkildsen 2011
-
- Torkildsen GL, Cockrum P, Meier E, Hammonds WM, Silverstein B, Silverstein S. Evaluation of clinical efficacy and safety of tobramycin/dexamethasone ophthalmic suspension 0.3%/0.05% compared to azithromycin ophthalmic solution 1% in the treatment of moderate to severe acute blepharitis/blepharoconjunctivitis. Current Medical Research and Opinion 2011;27(1):171‐8. - PubMed
Varni 2001
-
- Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical Care 2001;39(8):800‐12. - PubMed
Viswalingam 2005
Wolthers 2011
-
- Wolthers OD. Growth suppression caused by corticosteroid eye drops. Journal of Pediatric Endocrinology and Metabolism 2011;24(5‐6):393‐4. - PubMed
Wong 2010
-
- Wong IB, Nischal KK. Managing a child with an external ocular disease. Journal of AAPOS 2010;14(1):68‐77. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous