Genetic Modification of Human Peripheral Blood Aspirates Using Recombinant Adeno-Associated Viral Vectors for Articular Cartilage Repair with a Focus on Chondrogenic Transforming Growth Factor-β Gene Delivery
- PMID: 28170175
- PMCID: PMC5442727
- DOI: 10.5966/sctm.2016-0149
Genetic Modification of Human Peripheral Blood Aspirates Using Recombinant Adeno-Associated Viral Vectors for Articular Cartilage Repair with a Focus on Chondrogenic Transforming Growth Factor-β Gene Delivery
Abstract
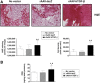
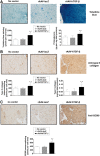
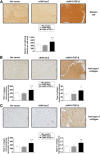
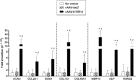
Transplantation of genetically modified peripheral blood aspirates that carry chondrogenically competent progenitor cells may offer new, convenient tools to treat articular cartilage lesions compared with the more complex and invasive application of bone marrow concentrates or of bone marrow-derived mesenchymal stem cells. Here, we show that recombinant adeno-associated viral (rAAV) vectors are powerful gene vehicles capable of successfully targeting primary human peripheral blood aspirates in a stable and safe manner, allowing for an efficient and long-term transgene expression in such samples (up to 63 days with use of a lacZ reporter gene and for at least 21 days with application of the pleiotropic, chondrogenic factor transforming growth factor-β [TGF-β]). rAAV-mediated overexpression of TGF-β enhanced both the proliferative and metabolic properties of the peripheral blood aspirates, also increasing the chondrogenic differentiation processes in these samples. Hypertrophy and osteogenic differentiation events were also activated by production of TGF-β via rAAV, suggesting that translation of the current approach in vivo will probably require close regulation of expression of this candidate gene. However, these results support the concept of directly modifying peripheral blood as a novel approach to conveniently treat articular cartilage lesions in patients. Stem Cells Translational Medicine 2017;6:249-260.
Keywords: Cartilage repair; Gene therapy; Peripheral blood aspirates; Transforming growth factor-β; rAAV vectors.
© 2016 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures



References
-
- Buckwalter JA Articular cartilage: Injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28:192–202 - PubMed
-
- Chong PP, Selvaratnam L, Abbas AA et al. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res. 2012;30:634–642 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

