Therapeutic Prospective of Infused Allogenic Cultured Mesenchymal Stem Cells in Traumatic Brain Injury Mice: A Longitudinal Proton Magnetic Resonance Spectroscopy Assessment
- PMID: 28170180
- PMCID: PMC5442758
- DOI: 10.5966/sctm.2016-0087
Therapeutic Prospective of Infused Allogenic Cultured Mesenchymal Stem Cells in Traumatic Brain Injury Mice: A Longitudinal Proton Magnetic Resonance Spectroscopy Assessment
Abstract
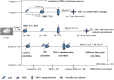
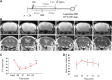
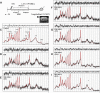
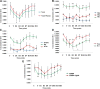
Improved therapeutic assessment of experimental traumatic brain injury (TBI), using mesenchymal stem cells (MSCs), would immensely benefit its therapeutic management. Neurometabolite patterns at injury site, measured with proton magnetic resonance spectroscopy (1H-MRS) after MSCs transplantation, may serve as a bio-indicator of the recovery mechanism. This study used in vivo magnetic resonance imaging and 1H-MRS to evaluate the therapeutic prospects of implanted MSCs at injury site in experimental mice longitudinally up to 21 days. Negative tissue contrast and cytotoxic edema formation were observed in susceptibility-based contrast (T2*) and an apparent diffusion coefficient map, respectively. Lesion site showed decreased N-acetylaspartate, total choline, myo-inositol, total creatine, glutamate-glutamine complex, and taurine neurometabolic concentrations by 1H-MRS investigation. There was a considerable decrease in locomotor activity, depression index, and cognitive index after TBI. It may, therefore, be inferred that MSC transplantation prompted recovery by decreasing negative signals and edema, restoring metabolites to baseline concentrations, and enhancing behavioral activity. Overall findings support the potential of MSC transplantation for the enhancement of endogenous neuroprotective responses, which may provide future clinical applications for translating laboratory research into therapeutic clinical advances. Stem Cells Translational Medicine 2017;6:316-329.
Keywords: Magnetic resonance imaging; Mesenchymal stem cells; Proton magnetic resonance spectroscopy; Stem cell therapy; Traumatic brain injury.
© 2016 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

