Enhanced Immunosuppression of T Cells by Sustained Presentation of Bioactive Interferon-γ Within Three-Dimensional Mesenchymal Stem Cell Constructs
- PMID: 28170190
- PMCID: PMC5442746
- DOI: 10.5966/sctm.2016-0044
Enhanced Immunosuppression of T Cells by Sustained Presentation of Bioactive Interferon-γ Within Three-Dimensional Mesenchymal Stem Cell Constructs
Abstract
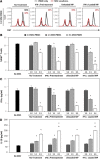
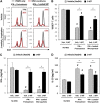
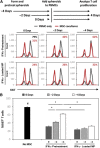
The immunomodulatory activity of mesenchymal stem/stromal cells (MSCs) to suppress innate and adaptive immune responses offers a potent cell therapy for modulating inflammation and promoting tissue regeneration. However, the inflammatory cytokine milieu plays a critical role in stimulating MSC immunomodulatory activity. In particular, interferon-γ (IFN-γ)-induced expression of indoleamine 2,3-dioxygenase (IDO) is primarily responsible for MSC suppression of T-cell proliferation and activation. Although pretreatment with IFN-γ is commonly used to prime MSCs for immunomodulatory activity prior to transplantation, the transient effects of pretreatment may limit the potential of MSCs to potently modulate immune responses. Therefore, the objective of this study was to investigate whether microparticle-mediated presentation of bioactive IFN-γ within three-dimensional spheroidal MSC aggregates could precisely regulate and induce sustained immunomodulatory activity. Delivery of IFN-γ via heparin-microparticles within MSC aggregates induced sustained IDO expression during 1 week of culture, whereas IDO expression by IFN-γ-pretreated MSC spheroids rapidly decreased during 2 days. Furthermore, sustained IDO expression induced by IFN-γ-loaded microparticles resulted in an increased and sustained suppression of T-cell activation and proliferation in MSC cocultures with CD3/CD28-activated peripheral blood mononuclear cells. The increased suppression of T cells by MSC spheroids containing IFN-γ-loaded microparticles was dependent on induction of IDO and supported by affecting monocyte secretion from pro- to anti-inflammatory cytokines. Altogether, microparticle delivery of IFN-γ within MSC spheroids provides a potent means of enhancing and sustaining immunomodulatory activity to control MSC immunomodulation after transplantation and thereby improve the efficacy of MSC-based therapies aimed at treating inflammatory and immune diseases. Stem Cells Translational Medicine 2017;6:223-237.
Keywords: Cellular; Immunomodulation; Indoleamine 2,3-dioxygenase; Mesenchymal stromal cells; Spheroids.
© 2016 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures




References
-
- Di Nicola M, Carlo‐Stella C, Magni M et al. Human bone marrow stromal cells suppress T‐lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99:3838–3843. - PubMed
-
- Krampera M, Glennie S, Dyson J et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen‐specific T cells to their cognate peptide. Blood 2003;101:3722–3729. - PubMed
-
- Corcione A, Benvenuto F, Ferretti E et al. Human mesenchymal stem cells modulate B‐cell functions. Blood 2006;107:367–372. - PubMed
-
- Nauta AJ, Kruisselbrink AB, Lurvink E et al. Mesenchymal stem cells inhibit generation and function of both CD34+‐derived and monocyte‐derived dendritic cells. J Immunol 2006;177:2080–2087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

