Concise Review: Hematopoietic Stem Cell Origins: Lessons from Embryogenesis for Improving Regenerative Medicine
- PMID: 28170201
- PMCID: PMC5442726
- DOI: 10.5966/sctm.2016-0110
Concise Review: Hematopoietic Stem Cell Origins: Lessons from Embryogenesis for Improving Regenerative Medicine
Abstract
Hematopoietic stem cells (HSCs) have extensive regenerative capacity to replace all blood cell types, an ability that is harnessed in the clinic for bone marrow transplantation. Finding appropriate donors remains a major limitation to more extensive usage of HSC-based therapies. Derivation of patient-specific HSCs from pluripotent stem cells offers great promise to remedy this problem if scientists could crack the code on how to make robust, transplantable HSCs in a dish. Studies delving into the native origins of HSC production during embryonic development should supply the necessary playbook. This review presents recent discoveries from animal models, with a focus on zebrafish, and discusses the implications of these new advances in the context of prior knowledge. The focus is on the latest research exploring the role of epigenetic regulation, signaling pathways, and niche components needed for proper HSC formation. These studies provide new directions that should be explored for de novo generation and expansion of HSCs for regenerative therapies. Stem Cells Translational Medicine 2017;6:60-67.
Keywords: Bone marrow transplant; Developmental biology; Embryo; Hematopoietic stem cells.
© 2016 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures

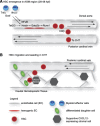
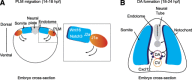
References
-
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 1996;86:897–906. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

